Merck Sharp & Dohme & Anr. v Glenmark Pharmaceuticals Ltd
|
|
|
- Harold Baldwin
- 6 years ago
- Views:
Transcription
1 BIOTECH BUZZ International Subcommittee December 2015 Contributor: Archana Shanker Changing trends in Indian patent enforcement In the history of the Patent Litigation in India, at least since 1970, only two cases have gone to trial. The two decisions in the Merck and Roche cases are certainly reassuring for patent owners. Merck Sharp & Dohme & Anr. v Glenmark Pharmaceuticals Ltd On 7 th October, 2015, India got its first judgment in 45 years, in the Merck Sharp & Dohme & Anr. v Glenmark Pharmaceuticals Ltd., where the Delhi High Court granted a permanent injunction against Glenmark for the infringement of MSD s patent for Sitagliptin. The highlights of this case before the High Court and Supreme Court had many firsts, namely: - For the first time, the Supreme Court held that IP litigation is commercial litigation; - That commercial litigation needs to be disposed of on an expedited basis which is vital for national interest. In the MSD case, in less than five months from the ordering of an expedited trial, the Judgment had been finally delivered by the Hon ble Delhi High Court; - The first permanent injunction ever granted to a patent owner;
2 Briefly on the facts of the case, the MSD has a composition of matter patent for Sitagliptin and its pharmaceutically acceptable salts, which is a DPP-IV Inhibitor essentially used for treatment of Type-II diabetes. MSD had separately applied for a salt patent for Sitagliptin Phosphate Monohydrate (SPM) which due to the unique provision of Section 3(d), was abandoned. Glenmark took the defense that one patent is for a product and the filing of a subsequent application for SPM, is an admission by the MSD, that SPM is not claimed or covered in the basic patent and the abandonment of the SPM application put SPM in public domain. Some of the highlights of this case are: - A consideration (even if it is minimal i.e. Re. 1 or USD 1) for a license agreement would not be a ground to challenge its authenticity. - In highly technical matters involving chemical compounds in the medical field, the Court held that the opinion of the experts is critical. The Court found Plaintiffs independent expert witness to be a more trustworthy and reliable witness in the chemical, biological and medicinal field than Defendant s independent expert witness - On infringement, the court held that the use of Sitagliptin in the defendant s infringing products, by itself, amounts to infringement of suit patent, i.e. use of the Sitagliptin free base in SPM tablet. - The Court also held that the plaintiffs had succeeded in proving that suit patent discloses SPM generically and that Sitagliptin free base is also disclosed. It is the Sitagliptin free base which is the DPP-IV inhibitor and phosphate salt is used for delivery of Sitagliptin in the body. SPM has improved physical and chemical characteristics, but the active moiety is Sitagliptin. - On validity, the Court held that the onus to establish obviousness of the suit patent over prior art was on the Defendant which it failed to discharge by leading positive evidence. In fact, the prior art search by the defendants witness was a hindsight analysis, which is impermissible under law. A mere comparison of chemical structure is not sufficient, i.e. picking up parts of
3 chemical structures of different patents and clubbing them, keeping in mind the molecule structure of the suit patent, would be hindsight analysis. - On industrial application, the court held that at the time when a patent is granted, it is not necessary to have the product manufactured. If the invention can be commercialized subsequently, it is sufficient. That the compounds formed or substances derived from the suit patent would be a product within the meaning of the Act. Further the Court held that the Plaintiffs drug does not lack industrial applicability as it has been successfully worked for treatment of diabetes. - On insufficiency and Markush claims (Broad Claiming), the court took note of the inventor s testimony that billions of compounds may seem a very large number but the chemical space is infinite. The court further held that Markush claims of the suit patent were no different from the Markush claims of other patent documents including Glenmark s patents. - On a section 8 violation, the Court agreed with the ratio laid down by the Division Bench in Sukesh Bhel & Anr. v Koninklijke Phillips Electronics, that the power of the Court to revoke a patent on the ground of a Section 8 violation is discretionary. In such cases, revocation would only follow if the Court is of the view that omission to furnish the information was deliberate. - On public interest the Court held that public interest is irrelevant as Sitagliptin is not the only DPP-IV inhibitor drug available for treatment of type II diabetes in the market. There are several others, including Defendant s teneligliptin. The court also stated that the sale of generic drug at a lower price cannot be a ground to decline injunction against a competitor Defendant who has been infringing the Plaintiffs patent. Hoffman La Roche vs Cipla Then on 27 th November 2015 / 8 th December 2015, came the order of the Division Bench (DB) in the Hoffman La Roche vs Cipla reversing the decision of the trial court (Single Judge) that held the basic patent of Roche for Tarceva as being valid but not infringed. The DB upheld the patent validity and held infringement of the patent of
4 Roche by Cipla. However, in view of the limited term remaining of the patent, the court didn t grant an injunction. Instead, the DB sent the matter back to the trial court for calculation of damages. The major findings of the DB in relation to the statutory provisions are as follows: 1. On the application of Section 3(d), the DB held that Section 3 is an exclusionary clause that lays down a threshold for patent eligibility and if the subject matter falls within the scope of Section 3, an analysis under Section 2(1)(j) need not be employed as it will be rejected at the threshold. The DB further held that Section 3(d) recognizes incremental innovations in pharmaceutical patents and does not in any manner relate to the concepts of evergreening or patent extension. 2. With regard to one patent one product, the DB held that a product patent protects the product in any form however it is made, or however it is formulated and that the rejection of a subsequent patent(in this case for a polymorphic form of Erlotinib Hydrochloride) under Section 3(d) cannot doubly penalize an innovator. In other words, when a patent application is rejected on the grounds that it does not meet the threshold of patent eligibility, it does not effectively permit the manufacturers of the said polymorph from being deemed non-infringers under Section 48. Thus, Section 3(d) cannot be interpreted as constituting a defence to infringement. 3. The DB also laid down the principles of claim construction and held that patent infringement analysis entails two steps: (a) Determine the meaning and scope of the patent claims asserted to be infringed; (b) Compare the properly construed claim with the device accused of infringing. Test of infringement is to compare claims with the infringing product. The test is not to compare patentee s product with the infringing product. 4. The DB introduced concepts like 'inter'-molecular and 'intra'-molecular concept and held that the claim for Erlotinib hydrochloride, nowhere mentions any polymorphic form. The chemical structure describes the manner in which each molecule of the compound exists. It is not an 'inter'-molecular concept but an 'intra'-molecular concept. Irrespective of the polymorphic
5 form of Erlotinib Hydrochloride, it would have the same chemical structure as contained in Claim 1 of the suit patent and is therefore subsumed within claim 1 of the suit patent. 5. The Court also struck a distinction between commercial utility and patent utility and recognized that at the time the inventions are invented, they may not be commercially the most viable for immediate marketing. 6. The court further held that a claim must be interpreted on its own language and resort cannot be had to subsequent statements/documents either to enlarge or to narrow the scope. 7. In relation to submission of X-ray diffraction for establishing infringement of compound patents, the court held that XRD is used to determine crystalline structure of a compound which is not an accurate method to ascertain product patent infringement of new chemical entities/compounds. X-ray diffraction data is not relevant in the present case, as the issue is not whether Roche and Cipla's products are identical but whether Cipla's product is covered in the claims of Roche's patent. Roche s patent is not for a polymorph. 8. On use of Polymorph B in the infringing product, the court held that it would first involve the preparation of Erlotinib Hydrochloride itself and therefore the use and manufacture of Erlotinib Hydrochloride amounts to infringement. 9. On section 8 violation, the court held that the patentee s compliance with Section 8 is mandatory but the power of the Court to revoke a patent under Section 64 where the non-compliance of Section 8 is a mere clerical or bona fide error is directory as indicated by the use of the word may. 10. In relation to obviousness, the court the following findings: a. Obviousness has to be tested at the priority date of the patent under challenge; b. To test obviousness, the first test required to be applied is to see who is an ordinary person skilled in art and what its characteristics are; c. Hindsight analysis is not permissible
6 d. Patent challenger must demonstrate that the selection of lead compound based on its promising useful properties; e. Test of obviousness involves identifying POSA, inventive concept, common general knowledge, identify difference between the matter cited and alleged invention and whether such differences are obvious to POSA and ruling out hindsight approach. f. For obviousness, besides structural similarity there should be a reason or motivation shown in the prior art to make the particular structural change. These two orders of the Delhi High Court have reinforced our faith in the judiciary for times to come. Nothing herein should be construed as legal advice or legal representation. Click here for an expanded disclaimer. Archana Shanker, Senior Partner & Head (Patents & Designs) - Anand and Anand, India archana@anandandanand.com
Ritushka Negi Remfry & Sagar, Partner
 Ritushka Negi Remfry & Sagar, Partner NO HOLDS BARRED KEY TAKEAWAYS FROM RECENT PATENT DECISIONS IN INDIA RITUSHKA NEGI November 21, 2016 www.remfry.com 3 Administrative & Judicial Hierarchy Supreme Court
Ritushka Negi Remfry & Sagar, Partner NO HOLDS BARRED KEY TAKEAWAYS FROM RECENT PATENT DECISIONS IN INDIA RITUSHKA NEGI November 21, 2016 www.remfry.com 3 Administrative & Judicial Hierarchy Supreme Court
Prathiba M. Singh President, APAA (Indian Group)
 Prathiba M. Singh President, APAA (Indian Group) Section 108 relates to relief in a suit for infringement Section 108(1) provides for Damages or Account of Profits At the option of the Plaintiff Section
Prathiba M. Singh President, APAA (Indian Group) Section 108 relates to relief in a suit for infringement Section 108(1) provides for Damages or Account of Profits At the option of the Plaintiff Section
IN THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : SUIT FOR INJUNCTION Date of decision: 5th April, CS(OS) 586/2013
 IN THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : SUIT FOR INJUNCTION Date of decision: 5th April, 2013. CS(OS) 586/2013 MERCK SHARP AND DOHME CORPORATION & ANR...Plaintiffs Through: Mr. Parag P. Tripathi,
IN THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : SUIT FOR INJUNCTION Date of decision: 5th April, 2013. CS(OS) 586/2013 MERCK SHARP AND DOHME CORPORATION & ANR...Plaintiffs Through: Mr. Parag P. Tripathi,
CORAM: HON'BLE MR. JUSTICE PRADEEP NANDRAJOG HON'BLE MS. JUSTICE MUKTA GUPTA O R D E R %
 $~ * IN THE HIGH COURT OF DELHI AT NEW DELHI + RFA(OS) 92/2012 F.HOFFMANN-LA ROCHE LTD & ANR.... Petitioners Represented by: Mr.Sudhir Chandra, Sr.Advocate instructed by Mr.Shrawan Chopra, Advocate versus
$~ * IN THE HIGH COURT OF DELHI AT NEW DELHI + RFA(OS) 92/2012 F.HOFFMANN-LA ROCHE LTD & ANR.... Petitioners Represented by: Mr.Sudhir Chandra, Sr.Advocate instructed by Mr.Shrawan Chopra, Advocate versus
From The Editor s Desk
 From The Editor s Desk Dear Reader, Welcome to the fourth edition of the "IP India News"! We have entered a new year - here is wishing you a very happy new year and happy reading too. Shortly after entering
From The Editor s Desk Dear Reader, Welcome to the fourth edition of the "IP India News"! We have entered a new year - here is wishing you a very happy new year and happy reading too. Shortly after entering
IN THE HIGH COURT OF DELHI AT NEW DELHI FAO (OS) 188/2008 F.HOFFMANN-LA ROCHE LTD. & ANR
 IN THE HIGH COURT OF DELHI AT NEW DELHI FAO (OS) 188/2008 F.HOFFMANN-LA ROCHE LTD. & ANR versus Date of decision: April 24 th 2009... Appellants Through Dr. A.M. Singhvi, Senior Advocate, Mr. Parag. P.
IN THE HIGH COURT OF DELHI AT NEW DELHI FAO (OS) 188/2008 F.HOFFMANN-LA ROCHE LTD. & ANR versus Date of decision: April 24 th 2009... Appellants Through Dr. A.M. Singhvi, Senior Advocate, Mr. Parag. P.
4. COMPARISON OF THE INDIAN PATENT LAW WITH THE PATENT LAWS IN U.S., EUROPE AND CHINA
 4. COMPARISON OF THE INDIAN PATENT LAW WITH THE PATENT LAWS IN U.S., EUROPE AND CHINA Provisions of the Indian patent law were compared with the relevant provisions of the patent laws in U.S., Europe and
4. COMPARISON OF THE INDIAN PATENT LAW WITH THE PATENT LAWS IN U.S., EUROPE AND CHINA Provisions of the Indian patent law were compared with the relevant provisions of the patent laws in U.S., Europe and
LEGAL DEVELOPMENTS. The important legal updates from the previous quarter are summarized below: Trade Marks Rules, 2017 Notified
 z This Newsletter brings to you the IP updates during the first quarter of this year. The first quarter saw remarkable changes in trademark practice and procedure in India. With substantial changes in
z This Newsletter brings to you the IP updates during the first quarter of this year. The first quarter saw remarkable changes in trademark practice and procedure in India. With substantial changes in
Intellectual Property and crystalline forms. How to get a European Patent on crystalline forms?
 Intellectual Property and crystalline forms How to get a European Patent on crystalline forms? Ambrogio Usuelli Chief-Examiner European Patent Office, Munich, Germany Bologna, 19th January 2012 Sponsor:
Intellectual Property and crystalline forms How to get a European Patent on crystalline forms? Ambrogio Usuelli Chief-Examiner European Patent Office, Munich, Germany Bologna, 19th January 2012 Sponsor:
Measures for Expediting Patent Examination in India. By Dr. Rajeshkumar H. Acharya
 Measures for Expediting Patent Examination in India By Dr. Rajeshkumar H. Acharya Indian phase entry time line 2 Do s: PCT National Phase Application In India Conventional Application In India Within thirty
Measures for Expediting Patent Examination in India By Dr. Rajeshkumar H. Acharya Indian phase entry time line 2 Do s: PCT National Phase Application In India Conventional Application In India Within thirty
IN THE HIGH COURT OF DELHI AT NEW DELHI. CS (OS) No of Versus CORAM: JUSTICE S. MURALIDHAR O R D E R
 IN THE HIGH COURT OF DELHI AT NEW DELHI CS (OS) No. 2206 of 2012 KONINKLIJKE PHILIPS ELECTRONICS N.V.... Plaintiff Through: Mr. Sudhir Chandra, Senior Advocate with Mr. Pravin Anand, Ms. Vaishali Mittal,
IN THE HIGH COURT OF DELHI AT NEW DELHI CS (OS) No. 2206 of 2012 KONINKLIJKE PHILIPS ELECTRONICS N.V.... Plaintiff Through: Mr. Sudhir Chandra, Senior Advocate with Mr. Pravin Anand, Ms. Vaishali Mittal,
A chapter called controversy: breaking down the Delhi high court appellate bench verdict in F Hoffmann-La Roche v Cipla Ltd
 Queen Mary Journal of Intellectual Property, Vol. 6 No. 2, pp. 260 272 A chapter called controversy: breaking down the Delhi high court appellate bench verdict in F Hoffmann-La Roche v Cipla Ltd Eashan
Queen Mary Journal of Intellectual Property, Vol. 6 No. 2, pp. 260 272 A chapter called controversy: breaking down the Delhi high court appellate bench verdict in F Hoffmann-La Roche v Cipla Ltd Eashan
BE it enacted by Parliament in the Fifty-sixth Year of the Republic of India as follows:-
 ~ THE PATENTS (AMENDMENT) ACT, 2005 # NO. 15 OF 2005 $ [4th April, 2005] + An Act further to amend the Patents Act, 1970. BE it enacted by Parliament in the Fifty-sixth Year of the Republic of India as
~ THE PATENTS (AMENDMENT) ACT, 2005 # NO. 15 OF 2005 $ [4th April, 2005] + An Act further to amend the Patents Act, 1970. BE it enacted by Parliament in the Fifty-sixth Year of the Republic of India as
6 th India IP IPR Summit 23 Feb 2009
 Obviousness Under India Patent Laws 6 th India IP IPR Summit 23 Feb 2009 Naren Thappeta US Patent Attorney India Patent Agent Bangalore, India www.iphorizons.com 23/Feb/2009 2009 Naren Thappeta 1 Broad
Obviousness Under India Patent Laws 6 th India IP IPR Summit 23 Feb 2009 Naren Thappeta US Patent Attorney India Patent Agent Bangalore, India www.iphorizons.com 23/Feb/2009 2009 Naren Thappeta 1 Broad
EMERGING IP RIGHTS. Country Report, India. D. Calab Gabriel
 RECENT DEVELOPMENT AND IMPORTANT DECISIONS IN INDIAN PATENT LAW ASIAN PATENT ATTORNEYS ASSOCIATION 63 rd Council Meeting Penang, Malaysia 8 th to 11 th November, 2014 EMERGING IP RIGHTS Country Report,
RECENT DEVELOPMENT AND IMPORTANT DECISIONS IN INDIAN PATENT LAW ASIAN PATENT ATTORNEYS ASSOCIATION 63 rd Council Meeting Penang, Malaysia 8 th to 11 th November, 2014 EMERGING IP RIGHTS Country Report,
The Patents (Amendment) Act,
 !"# The Patents (Amendment) Act, 2005 1 [NO. 15 OF 2005] CONTENTS [April 4, 2005] Sections Sections 1. Short title and commencement 40. Amendment of Section 57 2. Amendment of Section 2 41. Substitution
!"# The Patents (Amendment) Act, 2005 1 [NO. 15 OF 2005] CONTENTS [April 4, 2005] Sections Sections 1. Short title and commencement 40. Amendment of Section 57 2. Amendment of Section 2 41. Substitution
November Contents. Article Willful or deliberate suppression standard under Section 8 of the Patents Act. Ratio Decidendi News Nuggets
 An e-newsletter from Lakshmikumaran & Sridharan, New Delhi, India November 2014 / Issue 40 Contents Article Willful or deliberate suppression standard under Section 8 of the Patents Act Ratio Decidendi
An e-newsletter from Lakshmikumaran & Sridharan, New Delhi, India November 2014 / Issue 40 Contents Article Willful or deliberate suppression standard under Section 8 of the Patents Act Ratio Decidendi
ASIAN PATENT ATTORNEYS ASSOCIATION. 62 nd Council Meeting. Hanoi, Vietnam. Patent Committee Report: INDIA. Hari Subramaniam, Neeti Dewan, Sanjay Kumar
 ASIAN PATENT ATTORNEYS ASSOCIATION 62 nd Council Meeting Hanoi, Vietnam Patent Committee Report: INDIA Hari Subramaniam, Neeti Dewan, Sanjay Kumar 1 India: Patents 2013 There have been no changes in statutory
ASIAN PATENT ATTORNEYS ASSOCIATION 62 nd Council Meeting Hanoi, Vietnam Patent Committee Report: INDIA Hari Subramaniam, Neeti Dewan, Sanjay Kumar 1 India: Patents 2013 There have been no changes in statutory
ASIAN PATENT ATTORNEYS ASSOCIATION
 ASIAN PATENT ATTORNEYS ASSOCIATION 63 rd Council Meeting Penang, Malaysia Patent Committee Report: INDIA Hari Subramaniam & Calab Gabriel 1 India: Patents 2014 There have been several changes in statutory
ASIAN PATENT ATTORNEYS ASSOCIATION 63 rd Council Meeting Penang, Malaysia Patent Committee Report: INDIA Hari Subramaniam & Calab Gabriel 1 India: Patents 2014 There have been several changes in statutory
THE PATENTS NEWSLETTER
 THE PATENTS NEWSLETTER In the whirlwind of events marking the patent landscape in India, we strive to keep you updated with our regular editions of the Patents Newsletter and bring you patents news from
THE PATENTS NEWSLETTER In the whirlwind of events marking the patent landscape in India, we strive to keep you updated with our regular editions of the Patents Newsletter and bring you patents news from
SPC system simple, transparent and easy to apply? By Peter Damerell, Ayesha Raghib and William Hillson Powell Gilbert LLP
 SPC system simple, transparent and easy to apply? By Peter Damerell, Ayesha Raghib and William Hillson Powell Gilbert LLP The strength and depth of our intellectual property expertise is second to none,
SPC system simple, transparent and easy to apply? By Peter Damerell, Ayesha Raghib and William Hillson Powell Gilbert LLP The strength and depth of our intellectual property expertise is second to none,
The Royal Society of Chemistry IP Law Case Seminar: 2017 in the U.S.
 Finnegan, Henderson, Farabow, Garrett & Dunner, LLP The Royal Society of Chemistry IP Law Case Seminar: 2017 in the U.S. Anthony C. Tridico, Ph.D. 2017 1 Agenda U.S. Supreme Court news 2017 U.S. Court
Finnegan, Henderson, Farabow, Garrett & Dunner, LLP The Royal Society of Chemistry IP Law Case Seminar: 2017 in the U.S. Anthony C. Tridico, Ph.D. 2017 1 Agenda U.S. Supreme Court news 2017 U.S. Court
Examining Patent Enforcement and Litigation in India from A Development Perspective A study
 Examining Patent Enforcement and Litigation in India from A Development Perspective A study Ayyappan Palanissamy + School of Business and Design, Swinburne University of Technology Sarawak, Kuching, Malaysia
Examining Patent Enforcement and Litigation in India from A Development Perspective A study Ayyappan Palanissamy + School of Business and Design, Swinburne University of Technology Sarawak, Kuching, Malaysia
The Judgment can be accessed here at the website of the Delhi High Court. The Judgment can also be accessed here at India Kanoon website.
 The Judgment can be accessed here at the website of the Delhi High Court. The Judgment can also be accessed here at India Kanoon website. The Facts: The brief facts of the case are as follows: The Plaintiff
The Judgment can be accessed here at the website of the Delhi High Court. The Judgment can also be accessed here at India Kanoon website. The Facts: The brief facts of the case are as follows: The Plaintiff
patents grant only the right to stop others from making, using and selling the invention
 1 I. What is a Patent? A patent is a limited right granted by a government (all patents are limited by country) that allows the inventor to stop other people or companies from making, using or selling
1 I. What is a Patent? A patent is a limited right granted by a government (all patents are limited by country) that allows the inventor to stop other people or companies from making, using or selling
How patents work An introduction for law students
 How patents work An introduction for law students 1 Learning goals The learning goals of this lecture are to understand: the different types of intellectual property rights available the role of the patent
How patents work An introduction for law students 1 Learning goals The learning goals of this lecture are to understand: the different types of intellectual property rights available the role of the patent
Second medical use or indication claims. Mr. Antonio Ray ORTIGUERA Angara Abello Concepcion Regala & Cruz Law Offices Philippines
 Question Q238 National Group: Title: Contributors: Reporter within Working Committee: PHILIPPINES Second medical use or indication claims Mr. Alex Ferdinand FIDER Mr. Antonio Ray ORTIGUERA Angara Abello
Question Q238 National Group: Title: Contributors: Reporter within Working Committee: PHILIPPINES Second medical use or indication claims Mr. Alex Ferdinand FIDER Mr. Antonio Ray ORTIGUERA Angara Abello
India Patent Act, 2003 Updated till March 11th, 2015
 India Patent Act, 2003 Updated till March 11th, 2015 TABLE OF CONTENTS CHAPTER I PRELIMINARY 1. Short title, extent and commencement. 2. Definitions and interpretation. CHAPTER II INVENTIONS NOT PATENTABLE
India Patent Act, 2003 Updated till March 11th, 2015 TABLE OF CONTENTS CHAPTER I PRELIMINARY 1. Short title, extent and commencement. 2. Definitions and interpretation. CHAPTER II INVENTIONS NOT PATENTABLE
Suzannah K. Sundby. canady + lortz LLP. David Read. Differences between US and EU Patent Laws that Could Cost You and Your Startup.
 Differences between US and EU Patent Laws that Could Cost You and Your Startup Suzannah K. Sundby United States canady + lortz LLP Europe David Read UC Center for Accelerated Innovation October 26, 2015
Differences between US and EU Patent Laws that Could Cost You and Your Startup Suzannah K. Sundby United States canady + lortz LLP Europe David Read UC Center for Accelerated Innovation October 26, 2015
EUROPEAN GENERIC MEDICINES ASSOCIATION
 EUROPEAN GENERIC MEDICINES ASSOCIATION POSITION PAPER POSITION PAPER ON THE REVIEW OF DIRECTIVE 2004/48/EC ON THE ENFORCEMENT OF INTELLECTUAL PROPERTY RIGHTS JUNE 2011 EGA EUROPEAN GENERIC MEDICINES ASSOCIATION
EUROPEAN GENERIC MEDICINES ASSOCIATION POSITION PAPER POSITION PAPER ON THE REVIEW OF DIRECTIVE 2004/48/EC ON THE ENFORCEMENT OF INTELLECTUAL PROPERTY RIGHTS JUNE 2011 EGA EUROPEAN GENERIC MEDICINES ASSOCIATION
THE PATENTS ACT 1970
 THE PATENTS ACT 1970 (39 of 1970) An Act to amend and consolidate the law relating to patents. (19 th September, 1970) Be it enacted by Parliament in the twenty first year of the Republic of India as follows;-
THE PATENTS ACT 1970 (39 of 1970) An Act to amend and consolidate the law relating to patents. (19 th September, 1970) Be it enacted by Parliament in the twenty first year of the Republic of India as follows;-
For reprint orders, please contact Endo Pharmaceuticals Inc. v. Actavis, Inc. Alexandra Sklan*,1 & Takeshi S Komatani 2
 For reprint orders, please contact reprints@future-science.com International roundup of recently filed cases and noteworthy rulings Alexandra Sklan*,1 & Takeshi S Komatani 2 Endo Pharmaceuticals Inc. v.
For reprint orders, please contact reprints@future-science.com International roundup of recently filed cases and noteworthy rulings Alexandra Sklan*,1 & Takeshi S Komatani 2 Endo Pharmaceuticals Inc. v.
Selection Inventions the Inventive Step Requirement, other Patentability Criteria and Scope of Protection
 Question Q209 National Group: Title: Contributors: AIPPI Indonesia Selection Inventions the Inventive Step Requirement, other Patentability Criteria and Scope of Protection Arifia J. Fajra (discussed by
Question Q209 National Group: Title: Contributors: AIPPI Indonesia Selection Inventions the Inventive Step Requirement, other Patentability Criteria and Scope of Protection Arifia J. Fajra (discussed by
* IN THE HIGH COURT OF DELHI AT NEW DELHI. % Judgment reserved on: 24 th April, 2015 Judgment delivered on: 08 th October, 2015
 * IN THE HIGH COURT OF DELHI AT NEW DELHI % Judgment reserved on: 24 th April, 2015 Judgment delivered on: 08 th October, 2015 + FAO(OS) 220/2015 & CM Nos.7502/2015, 7504/2015 SERGI TRANSFORMER EXPLOSION
* IN THE HIGH COURT OF DELHI AT NEW DELHI % Judgment reserved on: 24 th April, 2015 Judgment delivered on: 08 th October, 2015 + FAO(OS) 220/2015 & CM Nos.7502/2015, 7504/2015 SERGI TRANSFORMER EXPLOSION
Time allowed : 3 hours Maximum marks : 100. Total number of questions : 6 Total number of printed pages : 8
 OPEN BOOK EXAMINATION Roll No... : 1 : 344 Time allowed : 3 hours Maximum marks : 100 Total number of questions : 6 Total number of printed pages : 8 NOTE : Answer ALL Questions. 1. Read the following
OPEN BOOK EXAMINATION Roll No... : 1 : 344 Time allowed : 3 hours Maximum marks : 100 Total number of questions : 6 Total number of printed pages : 8 NOTE : Answer ALL Questions. 1. Read the following
IN THE HIGH COURT OF DELHI AT NEW DELHI FAO (OS) 367/2007. Date of Decision : 08 TH FEBRUARY, 2008
 IN THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : Code of Civil Procedure FAO (OS) 367/2007 Date of Decision : 08 TH FEBRUARY, 2008 EUREKA FORBES LTD. & ANR.... Appellants Through : Mr. Valmiki Mehta,
IN THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : Code of Civil Procedure FAO (OS) 367/2007 Date of Decision : 08 TH FEBRUARY, 2008 EUREKA FORBES LTD. & ANR.... Appellants Through : Mr. Valmiki Mehta,
PRE-GRANT OPPOSITION POST-GRANT OPPOSITION
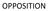 OPPOSITION TYPES OF OPPOSITION PRE-GRANT OPPOSITION [SEC 25(1)] POST-GRANT OPPOSITION [SEC. 25 (2)] REVOCATION[SECs 64 TO 66] GROUNDS FOR OPPOSITION UNDER SECTIONS 25(1) & 25 (2) That the applicant for
OPPOSITION TYPES OF OPPOSITION PRE-GRANT OPPOSITION [SEC 25(1)] POST-GRANT OPPOSITION [SEC. 25 (2)] REVOCATION[SECs 64 TO 66] GROUNDS FOR OPPOSITION UNDER SECTIONS 25(1) & 25 (2) That the applicant for
Second medical use or indication claims
 Question Q238 National Group: Title: Contributors: Reporter within Working Committee: Egyptian National Group Second medical use or indication claims Eman MOHEY, Gamal ABOU ALI Ahmed ABOU ALI Date: May
Question Q238 National Group: Title: Contributors: Reporter within Working Committee: Egyptian National Group Second medical use or indication claims Eman MOHEY, Gamal ABOU ALI Ahmed ABOU ALI Date: May
Are Your Chinese Patents At Risk?
 October 2004 Are Your Chinese Patents At Risk? Viagra, the anti-impotence drug made by Pfizer, generated about $1.7 billion in worldwide sales last year. Viagra s active ingredient is a substance called
October 2004 Are Your Chinese Patents At Risk? Viagra, the anti-impotence drug made by Pfizer, generated about $1.7 billion in worldwide sales last year. Viagra s active ingredient is a substance called
Second medical use or indication claims. Winnie Tham, Edmund Kok, Nicholas Ong
 Question Q238 National Group: Title: Contributors: Reporter within Working Committee: AIPPI SINGAPORE Second medical use or indication claims Winnie Tham, Edmund Kok, Nicholas Ong THAM, Winnie Date: 17
Question Q238 National Group: Title: Contributors: Reporter within Working Committee: AIPPI SINGAPORE Second medical use or indication claims Winnie Tham, Edmund Kok, Nicholas Ong THAM, Winnie Date: 17
Power of Attorneys Executed out of India - Requirement of Notarization & Evidentiary Value before Courts of India By
 Power of Attorneys Executed out of India - Requirement of Notarization & Evidentiary Value before Courts of India By *Vijay Pal Dalmia, Advocate & Partner Vaish Associates Advocates Email: vpdalmia@vaishlaw.com
Power of Attorneys Executed out of India - Requirement of Notarization & Evidentiary Value before Courts of India By *Vijay Pal Dalmia, Advocate & Partner Vaish Associates Advocates Email: vpdalmia@vaishlaw.com
PATENT, TRADEMARK & COPYRIGHT!
 A BNA s PATENT, TRADEMARK & COPYRIGHT! JOURNAL Reproduced with permission from BNA s Patent, Trademark & Copyright Journal, 81 PTCJ 36, 11/05/2010. Copyright 2010 by The Bureau of National Affairs, Inc.
A BNA s PATENT, TRADEMARK & COPYRIGHT! JOURNAL Reproduced with permission from BNA s Patent, Trademark & Copyright Journal, 81 PTCJ 36, 11/05/2010. Copyright 2010 by The Bureau of National Affairs, Inc.
T H E W O R L D J O U R N A L O N J U R I S T I C P O L I T Y. BOLAR EXEMPTION VS. DATA EXCLUSIVITY: RIGHT TO HEALTH vs RIGHT OF PATENT HOLDER
 BOLAR EXEMPTION VS. DATA EXCLUSIVITY: RIGHT TO HEALTH vs RIGHT OF PATENT HOLDER Rhea Roy Mammen M.S. Ramaiah College of Law, Bangalore Introduction Pharmaceutical Patent has seen an increasing conflict
BOLAR EXEMPTION VS. DATA EXCLUSIVITY: RIGHT TO HEALTH vs RIGHT OF PATENT HOLDER Rhea Roy Mammen M.S. Ramaiah College of Law, Bangalore Introduction Pharmaceutical Patent has seen an increasing conflict
The Patent Failure of Novartis with Gleevec
 1 The Patent Failure of Novartis with Gleevec The Indian Supreme Court s verdict on the Novartis patent application has garnered a lot of attention as having set a stringent standard of nonobviousness
1 The Patent Failure of Novartis with Gleevec The Indian Supreme Court s verdict on the Novartis patent application has garnered a lot of attention as having set a stringent standard of nonobviousness
* IN THE HIGH COURT OF DELHI AT NEW DELHI
 * IN THE HIGH COURT OF DELHI AT NEW DELHI + FAO(OS) No.16 OF 2014 Pronounced on:07.11.2014 MAJ. (RETD.) SUKESH BEHL & ANR.... Appellants Through: Mr. Ajay Sahni along with Mr.N.K. Bhardwaj, Mr. Dushyant
* IN THE HIGH COURT OF DELHI AT NEW DELHI + FAO(OS) No.16 OF 2014 Pronounced on:07.11.2014 MAJ. (RETD.) SUKESH BEHL & ANR.... Appellants Through: Mr. Ajay Sahni along with Mr.N.K. Bhardwaj, Mr. Dushyant
Intellectual Property Department Hong Kong, China. Contents
 Intellectual Property Department Hong Kong, China Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 3 Section 3: Experimental use and/or scientific research... 3 Section
Intellectual Property Department Hong Kong, China Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 3 Section 3: Experimental use and/or scientific research... 3 Section
REPUBLIC OF VANUATU BILL FOR THE PATENTS ACT NO. OF 1999
 REPUBLIC OF VANUATU BILL FOR THE PATENTS ACT NO. OF 1999 Arrangement of Sections PART 1 PRELIMINARY PROVISIONS 1. Interpretation PART 2 PATENTABILITY 2. Patentable invention 3. Inventions not patentable
REPUBLIC OF VANUATU BILL FOR THE PATENTS ACT NO. OF 1999 Arrangement of Sections PART 1 PRELIMINARY PROVISIONS 1. Interpretation PART 2 PATENTABILITY 2. Patentable invention 3. Inventions not patentable
Are the Patented Medicines (Notice of Compliance) Regulations Working?
 Are the Patented Medicines (Notice of Compliance) Regulations Working? Edward Hore Hazzard & Hore 141 Adelaide Street West, Suite 1002 Toronto, ON M5H 3L5 (416) 868-1340 edhore@hazzardandhore.com March
Are the Patented Medicines (Notice of Compliance) Regulations Working? Edward Hore Hazzard & Hore 141 Adelaide Street West, Suite 1002 Toronto, ON M5H 3L5 (416) 868-1340 edhore@hazzardandhore.com March
Rules for the Implementation of the Patent Law of the People's Republic of China
 Rules for the Implementation of the Patent Law of the People's Republic of China (Promulgated by Decree No. 306 of the State Council of the People's Republic of China on June 15, 2001, and revised according
Rules for the Implementation of the Patent Law of the People's Republic of China (Promulgated by Decree No. 306 of the State Council of the People's Republic of China on June 15, 2001, and revised according
LALL & SETHI ADVOCATES
 PATENT IN I. Filing of Application An application for patent can be made by completing and submitting a set of forms along with the prescribed fees with the Indian Patent Office (IPO). To file an application
PATENT IN I. Filing of Application An application for patent can be made by completing and submitting a set of forms along with the prescribed fees with the Indian Patent Office (IPO). To file an application
Experimental Use Exemption of Patent Infringement A Brief Comparison of China and the United States
 BIOTECH BUZZ International Subcommittee January 2015 Contributors: Li Feng, PhD, Jiancheng Jiang and Yuan Wang Experimental Use Exemption of Patent Infringement A Brief Comparison of China and the United
BIOTECH BUZZ International Subcommittee January 2015 Contributors: Li Feng, PhD, Jiancheng Jiang and Yuan Wang Experimental Use Exemption of Patent Infringement A Brief Comparison of China and the United
Patent Prosecution Update
 Patent Prosecution Update March 2012 Contentious Proceedings at the USPTO Under the America Invents Act by Rebecca M. McNeill The America Invents Act of 2011 (AIA) makes significant changes to contentious
Patent Prosecution Update March 2012 Contentious Proceedings at the USPTO Under the America Invents Act by Rebecca M. McNeill The America Invents Act of 2011 (AIA) makes significant changes to contentious
The World Intellectual Property Organization
 The World Intellectual Property Organization The World Intellectual Property Organization is an international organization dedicated to ensuring that the rights of creators and owners of intellectual property
The World Intellectual Property Organization The World Intellectual Property Organization is an international organization dedicated to ensuring that the rights of creators and owners of intellectual property
Switzerland. Esther Baumgartner Christoph Berchtold Simon Holzer Kilian Schärli Meyerlustenberger Lachenal. 1. Small molecules
 Esther Baumgartner Christoph Berchtold Simon Holzer Kilian Schärli Meyerlustenberger Lachenal 1. Small molecules 1.1 Product and process claims Classic drug development works with small, chemically manufactured
Esther Baumgartner Christoph Berchtold Simon Holzer Kilian Schärli Meyerlustenberger Lachenal 1. Small molecules 1.1 Product and process claims Classic drug development works with small, chemically manufactured
intellectual property law CARR ideas on Declaring dependence What s in a name? Get Reddy Working for statutory damages Intellectual Property Law
 ideas on intellectual property law in this issue year end 2004 Declaring dependence Dependent patent claims and the doctrine of equivalents What s in a name? Triagra loses battle for trademark rights Get
ideas on intellectual property law in this issue year end 2004 Declaring dependence Dependent patent claims and the doctrine of equivalents What s in a name? Triagra loses battle for trademark rights Get
Second medical use or indication claims
 Question Q238 National Group: Title: Contributors: Reporter within Working Committee: Bulgarian National Group Second medical use or indication claims Valentina NESHEVA Valentina NESHEVA Date: 16 May 2014
Question Q238 National Group: Title: Contributors: Reporter within Working Committee: Bulgarian National Group Second medical use or indication claims Valentina NESHEVA Valentina NESHEVA Date: 16 May 2014
People's Republic of Bangladesh THE PATENTS AND DESIGNS ACT ACT NO. II OF 1911 as amended by Act No. XV of 2003 Entry into force: May 13, 2003
 People's Republic of Bangladesh THE PATENTS AND DESIGNS ACT ACT NO. II OF 1911 as amended by Act No. XV of 2003 Entry into force: May 13, 2003 TABLE OF CONTENTS PRELIMINARY 1. Short title, extent and commencement
People's Republic of Bangladesh THE PATENTS AND DESIGNS ACT ACT NO. II OF 1911 as amended by Act No. XV of 2003 Entry into force: May 13, 2003 TABLE OF CONTENTS PRELIMINARY 1. Short title, extent and commencement
Patent Act, B.E (1979) As Amended until Patent Act (No.3), B.E (1999) Translation
 Patent Act, B.E. 2522 (1979) As Amended until Patent Act (No.3), B.E. 2542 (1999) Translation BHUMIBOL ADULYADEJ, REX. Given on the 11th day of March, B.E. 2522; Being the 34th year of the present Reign
Patent Act, B.E. 2522 (1979) As Amended until Patent Act (No.3), B.E. 2542 (1999) Translation BHUMIBOL ADULYADEJ, REX. Given on the 11th day of March, B.E. 2522; Being the 34th year of the present Reign
The Patented Medicines (Notice of Compliance) Regulations: What patents are eligible to be listed on the register?
 The Patented Medicines (Notice of Compliance) Regulations: What patents are eligible to be listed on the register? Edward Hore Hazzard & Hore 141 Adelaide Street West, Suite 1002 Toronto, ON M5H 3L5 (416)
The Patented Medicines (Notice of Compliance) Regulations: What patents are eligible to be listed on the register? Edward Hore Hazzard & Hore 141 Adelaide Street West, Suite 1002 Toronto, ON M5H 3L5 (416)
Procedures of Second Instance Related to Civil Disputes. over Patent Infringement
 Procedures of Second Instance Related to Civil Disputes over Patent Infringement 86 Procedures of Second Instance Related to Civil Disputes over Patent Infringement I. Trial System in China China practices
Procedures of Second Instance Related to Civil Disputes over Patent Infringement 86 Procedures of Second Instance Related to Civil Disputes over Patent Infringement I. Trial System in China China practices
Tools and Pitfalls Recent Decisions from the EPO Boards of Appeal 20 November 2014
 Tools and Pitfalls Recent Decisions from the EPO Boards of Appeal 20 November 2014 Presented by: Leythem A. Wall Overview Acceleration of Appeal Proceedings Double Patenting Admissibility of Appeals Added
Tools and Pitfalls Recent Decisions from the EPO Boards of Appeal 20 November 2014 Presented by: Leythem A. Wall Overview Acceleration of Appeal Proceedings Double Patenting Admissibility of Appeals Added
Remedies: Injunction and Damages. 1. General
 VI. Remedies: Injunction and Damages 1. General If infringement is found and validity of the patent is not denied by the court, then the patentee is entitled to the remedies of both injunction and damages
VI. Remedies: Injunction and Damages 1. General If infringement is found and validity of the patent is not denied by the court, then the patentee is entitled to the remedies of both injunction and damages
IPFocus LIFE SCIENCES 9TH EDITION WHEN IS POST-PUBLISHED EVIDENCE ACCEPTABLE? VALEA
 IPFocus LIFE SCIENCES 9TH EDITION WHEN IS POST-PUBLISHED EVIDENCE ACCEPTABLE? VALEA 2011 EPO: INVENTIVE STEP When is post-published evidence acceptable? Ronney Wiklund and Anette Romare of Valea discuss
IPFocus LIFE SCIENCES 9TH EDITION WHEN IS POST-PUBLISHED EVIDENCE ACCEPTABLE? VALEA 2011 EPO: INVENTIVE STEP When is post-published evidence acceptable? Ronney Wiklund and Anette Romare of Valea discuss
FINAL REPORT THE PATENTS AND DESIGNS ACT, INTRODUCTION PATENTS
 FINAL REPORT ON THE PATENTS AND DESIGNS ACT, 200----- INTRODUCTION PATENTS In England grants of monopoly rights to exploit an invention by the inventor date back to the Elizabethan (Queen Elizabeth I)
FINAL REPORT ON THE PATENTS AND DESIGNS ACT, 200----- INTRODUCTION PATENTS In England grants of monopoly rights to exploit an invention by the inventor date back to the Elizabethan (Queen Elizabeth I)
Case5:13-cv BLF Document140 Filed05/01/15 Page1 of 11 UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN JOSE DIVISION
 Case:-cv-00-BLF Document0 Filed0/0/ Page of UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN JOSE DIVISION GILEAD SCIENCES, INC., Plaintiff, v. MERCK & CO, INC., et al., Defendants. Case
Case:-cv-00-BLF Document0 Filed0/0/ Page of UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN JOSE DIVISION GILEAD SCIENCES, INC., Plaintiff, v. MERCK & CO, INC., et al., Defendants. Case
Demystifying India s Patent Regime
 Demystifying India s Patent Regime Pankaj Soni October 30, 2014 www.remfry.com 1 AGENDA A Snapshot of India s Enforcement System Recent Decisions Impacting Patent Prosecution Proof of Right Section 8 Reporting
Demystifying India s Patent Regime Pankaj Soni October 30, 2014 www.remfry.com 1 AGENDA A Snapshot of India s Enforcement System Recent Decisions Impacting Patent Prosecution Proof of Right Section 8 Reporting
Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law
 !!! Dangers for Access to Medicines in the Trans-Pacific Partnership Agreement: Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law ! Issue US TPPA Proposal Andean Community
!!! Dangers for Access to Medicines in the Trans-Pacific Partnership Agreement: Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law ! Issue US TPPA Proposal Andean Community
Through: Mr. Sandeep Sethi, Sr. Adv. with Mr. Gurpreet Singh, Mr. Nitish Jain & Mr. Jatin Sethi, Advs. Versus
 IN THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : CODE OF CRIMINAL PROCEDURE Date of decision: 29th January, 2014 LPA 548/2013, CMs No.11737/2013 (for stay), 11739/2013 & 11740/2013 (both for condonation
IN THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : CODE OF CRIMINAL PROCEDURE Date of decision: 29th January, 2014 LPA 548/2013, CMs No.11737/2013 (for stay), 11739/2013 & 11740/2013 (both for condonation
WIPO Conference on IP Dispute Resolution in Life Sciences 2016 Amanda K. Murphy, Ph.D.
 Finnegan Europe LLP WIPO Conference on IP Dispute Resolution in Life Sciences 2016 Amanda K. Murphy, Ph.D. 1 U.S. Judicial System U.S. Supreme Court Quasi- Judicial Federal Agencies Federal Circuit International
Finnegan Europe LLP WIPO Conference on IP Dispute Resolution in Life Sciences 2016 Amanda K. Murphy, Ph.D. 1 U.S. Judicial System U.S. Supreme Court Quasi- Judicial Federal Agencies Federal Circuit International
Port Adelaide District Hockey Club Inc. Constitution
 Port Adelaide District Hockey Club Inc Constitution Table of Contents Constitution 1 NAME...4 2 DEFINITIONS...4 3 OBJECTS OR PURPOSES OF THE CLUB...4 4 POWERS OF THE CLUB...4 5 MEMBERSHIP...5 5.1 Admission
Port Adelaide District Hockey Club Inc Constitution Table of Contents Constitution 1 NAME...4 2 DEFINITIONS...4 3 OBJECTS OR PURPOSES OF THE CLUB...4 4 POWERS OF THE CLUB...4 5 MEMBERSHIP...5 5.1 Admission
The Changing Face of U.S. Patent Litigation
 The Changing Face of U.S. Patent Litigation Presented by the IP Litigation Group of Simpson Thacher & Bartlett LLP October 2007 Background on Simpson Thacher Founded 1884 in New York City Now, over 750
The Changing Face of U.S. Patent Litigation Presented by the IP Litigation Group of Simpson Thacher & Bartlett LLP October 2007 Background on Simpson Thacher Founded 1884 in New York City Now, over 750
The Same Invention or Not the Same Invention? Thorsten Bausch
 The Same Invention or Not the Same Invention? Thorsten Bausch FICPI World Congress Munich 2010 CONTENTS The Same Invention or Not the Same Invention? Practical Problems The standard of sameness the skilled
The Same Invention or Not the Same Invention? Thorsten Bausch FICPI World Congress Munich 2010 CONTENTS The Same Invention or Not the Same Invention? Practical Problems The standard of sameness the skilled
NATIONAL COMPANY LAW APPELLATE TRIBUNAL, NEW DELHI
 NATIONAL COMPANY LAW APPELLATE TRIBUNAL, NEW DELHI Company Appeals (AT) No.101 to 105 of 2017 (arising out of Order dated 06.02.2017 passed by the National Company Law Tribunal, New Delhi in CP Nos. 16/152/2015,
NATIONAL COMPANY LAW APPELLATE TRIBUNAL, NEW DELHI Company Appeals (AT) No.101 to 105 of 2017 (arising out of Order dated 06.02.2017 passed by the National Company Law Tribunal, New Delhi in CP Nos. 16/152/2015,
TRADE MARKS ACT, 1999
 GOVERNMENT OF THE PEOPLE S REPUBLIC OF BANGLADESH A DRAFT BILL OF THE PROPOSED TRADE MARKS ACT, 1999 Prepared in the light of the complete report made by the Bangladesh Law Commission recommending promulgation
GOVERNMENT OF THE PEOPLE S REPUBLIC OF BANGLADESH A DRAFT BILL OF THE PROPOSED TRADE MARKS ACT, 1999 Prepared in the light of the complete report made by the Bangladesh Law Commission recommending promulgation
Law on Inventive Activity*
 Law on Inventive Activity* (of October 19, 1972, as amended by the Law of April 16, 1993) TABLE OF CONTENTS** Article Part I: General Provisions... 1 9 Part II: Inventions and Patents 1. Patents... 10
Law on Inventive Activity* (of October 19, 1972, as amended by the Law of April 16, 1993) TABLE OF CONTENTS** Article Part I: General Provisions... 1 9 Part II: Inventions and Patents 1. Patents... 10
Additional Features of New Patent Ordinance
 Additional Features of New Patent Ordinance Wednesday, January 19, 2005 08:00 IST Dr. Gopakumar G. Nair The Patent (Amendment) Ordinance 2004 and the Patent (Amendment) Rules 2005 notified on 26th December
Additional Features of New Patent Ordinance Wednesday, January 19, 2005 08:00 IST Dr. Gopakumar G. Nair The Patent (Amendment) Ordinance 2004 and the Patent (Amendment) Rules 2005 notified on 26th December
: 1 : Time allowed : 3 hours Maximum marks : 100. Total number of questions : 6 Total number of printed pages : 7
 OPEN BOOK EXAMINATION Roll No : 1 : NEW SYLLABUS Time allowed : 3 hours Maximum marks : 100 Total number of questions : 6 Total number of printed pages : 7 NOTE : Answer ALL Questions. 1. Read the case
OPEN BOOK EXAMINATION Roll No : 1 : NEW SYLLABUS Time allowed : 3 hours Maximum marks : 100 Total number of questions : 6 Total number of printed pages : 7 NOTE : Answer ALL Questions. 1. Read the case
The Korean Drug Approval-Patent Linkage System: A Comparison with the US Hatch-Waxman Act
 FEBRUARY 2015 The Korean Drug Approval-Patent Linkage System: A Comparison with the US Hatch-Waxman Act Authors: Ki Young Kim, Hyunsuk Jin, Samuel SungMok Lee Pursuant to the implementation of the Korea-US
FEBRUARY 2015 The Korean Drug Approval-Patent Linkage System: A Comparison with the US Hatch-Waxman Act Authors: Ki Young Kim, Hyunsuk Jin, Samuel SungMok Lee Pursuant to the implementation of the Korea-US
Current Status and Challenges concerning IP Litigation in China
 Current Status and Challenges concerning IP Litigation in China 2013 by Dr. Jiang Zhipei KING & WOOD MALLESONS 1 Current Status of IP Litigation in China 2 1.1 Statistics 3 1.1 Statistics The number of
Current Status and Challenges concerning IP Litigation in China 2013 by Dr. Jiang Zhipei KING & WOOD MALLESONS 1 Current Status of IP Litigation in China 2 1.1 Statistics 3 1.1 Statistics The number of
Supreme Court decision regarding the 5th Requirement of the Doctrine of
 Asamura NEWS Vol. 26 July 2018 Kenji Wada Attorney at Law Asamura Law Offices kwada@asamura.jp Mari Yuge Patent Attorney Chemical Department myuge@asamura.jp Hisashi Kanamori Patent Attorney Chemical Department
Asamura NEWS Vol. 26 July 2018 Kenji Wada Attorney at Law Asamura Law Offices kwada@asamura.jp Mari Yuge Patent Attorney Chemical Department myuge@asamura.jp Hisashi Kanamori Patent Attorney Chemical Department
An introduction to European intellectual property rights
 An introduction to European intellectual property rights Scott Parker Adrian Smith Simmons & Simmons LLP 1. Patents 1.1 Patentable inventions The requirements for patentable inventions are set out in Article
An introduction to European intellectual property rights Scott Parker Adrian Smith Simmons & Simmons LLP 1. Patents 1.1 Patentable inventions The requirements for patentable inventions are set out in Article
Second medical use or indication claims. [Please insert name last name in CAPITAL letters please]
![Second medical use or indication claims. [Please insert name last name in CAPITAL letters please] Second medical use or indication claims. [Please insert name last name in CAPITAL letters please]](/thumbs/82/85618671.jpg) Question Q238 National Group: Title: Contributors: Reporter within Working Committee: New Zealand Second medical use or indication claims Michael BROWN, Partner Helen BELLCHAMBERS, Associate A J Park [Please
Question Q238 National Group: Title: Contributors: Reporter within Working Committee: New Zealand Second medical use or indication claims Michael BROWN, Partner Helen BELLCHAMBERS, Associate A J Park [Please
Norway. Norway. By Rune Nordengen, Bull & Co Advokatfirma AS
 Norway By Rune Nordengen, Bull & Co Advokatfirma AS 1. What are the most effective ways for a European patent holder whose rights cover your jurisdiction to enforce its rights in your jurisdiction? Cases
Norway By Rune Nordengen, Bull & Co Advokatfirma AS 1. What are the most effective ways for a European patent holder whose rights cover your jurisdiction to enforce its rights in your jurisdiction? Cases
IP & IT Bytes. November Patents: jurisdiction and declaratory relief
 November 2016 IP & IT Bytes First published in the November 2016 issue of PLC Magazine and reproduced with the kind permission of the publishers. Subscription enquiries 020 7202 1200. Patents: jurisdiction
November 2016 IP & IT Bytes First published in the November 2016 issue of PLC Magazine and reproduced with the kind permission of the publishers. Subscription enquiries 020 7202 1200. Patents: jurisdiction
DHS Patentanwaltsgesellschaft mbh Munich. RECENT RULINGS OF THE EUROPEAN COURT OF JUSTICE ON SPCs
 Dr. Stefan Danner December 2011 German and European Patent Attorney danner@dhs-patent.de RECENT RULINGS OF THE EUROPEAN COURT OF JUSTICE ON SPCs In the last few months, the European Court of Justice (ECJ)
Dr. Stefan Danner December 2011 German and European Patent Attorney danner@dhs-patent.de RECENT RULINGS OF THE EUROPEAN COURT OF JUSTICE ON SPCs In the last few months, the European Court of Justice (ECJ)
Chemical Patent Practice. Course Syllabus
 Chemical Patent Practice Course Syllabus I. INTRODUCTION TO CHEMICAL PATENT PRACTICE: SETTING THE STAGE FOR DISCUSSING STRATEGIES FOR REDUCING RISK OF UNENFORCEABILITY AND ENHANCING CHANCES OF INFRINGEMENT,
Chemical Patent Practice Course Syllabus I. INTRODUCTION TO CHEMICAL PATENT PRACTICE: SETTING THE STAGE FOR DISCUSSING STRATEGIES FOR REDUCING RISK OF UNENFORCEABILITY AND ENHANCING CHANCES OF INFRINGEMENT,
INVALIDITY DEFENSE IN PATENT INFRINGEMENT LITIGATIONS IN JAPAN. July 25,2014 Chief Judge Ryuichi Shitara Intellectual Property High Court
 INVALIDITY DEFENSE IN PATENT INFRINGEMENT LITIGATIONS IN JAPAN July 25,2014 Chief Judge Ryuichi Shitara Intellectual Property High Court INVALIDATION TRIAL AT JPO Article 123of the Patent Act (2) Any person
INVALIDITY DEFENSE IN PATENT INFRINGEMENT LITIGATIONS IN JAPAN July 25,2014 Chief Judge Ryuichi Shitara Intellectual Property High Court INVALIDATION TRIAL AT JPO Article 123of the Patent Act (2) Any person
2013 International Series Korea U.S. IP Judicial Conference. Patentability of Chemical/Pharmaceutical Inventions. Isomers/Enantiomers
 2013 International Series Korea U.S. IP Judicial Conference Patentability of Chemical/Pharmaceutical Inventions October 22, 2013 Nicholas M. Cannella, Esq. 1 Chemical Structure: Stereochemistry The three-dimensional
2013 International Series Korea U.S. IP Judicial Conference Patentability of Chemical/Pharmaceutical Inventions October 22, 2013 Nicholas M. Cannella, Esq. 1 Chemical Structure: Stereochemistry The three-dimensional
LAWS OF MALAWI PATENTS CHAPTER 49:02 CURRENT PAGES
 PATENTS CHAPTER 49:02 PAGE CURRENT PAGES L.R.O. 1 4 1/1986 5 10 1/1968 11 12 1/1986 13 64 1/1968 65 68 1/1970 69-86 1/1968 87 88 1/1970 89 90 1/1993 91 108 1/1968 109 112 1/1993 112a 1/1993 113 114 1/1968
PATENTS CHAPTER 49:02 PAGE CURRENT PAGES L.R.O. 1 4 1/1986 5 10 1/1968 11 12 1/1986 13 64 1/1968 65 68 1/1970 69-86 1/1968 87 88 1/1970 89 90 1/1993 91 108 1/1968 109 112 1/1993 112a 1/1993 113 114 1/1968
Patent Term Extensions in Taiwan
 This article was published in the Markgraf Ergänzende Schutzzertifikate - Patent Term Extensions on 2015. Patent Term Extensions in Taiwan I. Introduction Ruth Fang, Lee and Li Attorneys at Law The patent
This article was published in the Markgraf Ergänzende Schutzzertifikate - Patent Term Extensions on 2015. Patent Term Extensions in Taiwan I. Introduction Ruth Fang, Lee and Li Attorneys at Law The patent
Dawn of an English Doctrine of Equivalents: immaterial variants infringe
 Dawn of an English Doctrine of Equivalents: immaterial variants infringe November 2017 The Supreme Court reinvents patent infringement The Supreme Court s landmark judgment in Actavis v Eli Lilly is a
Dawn of an English Doctrine of Equivalents: immaterial variants infringe November 2017 The Supreme Court reinvents patent infringement The Supreme Court s landmark judgment in Actavis v Eli Lilly is a
INDIAN PATENTS. Request for Examination. 48 months from priority*
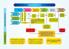 INDIAN PATENTS INDIAN PATENT PROSECUTION ASA FACILITATION Direct filing (Priority application) 31 months from Priority (PCT Route) Filing 12 months from Basic Application (Convention Route) 18 months from
INDIAN PATENTS INDIAN PATENT PROSECUTION ASA FACILITATION Direct filing (Priority application) 31 months from Priority (PCT Route) Filing 12 months from Basic Application (Convention Route) 18 months from
PATENT REEXAMINATION BOARD OF THE STATE INTELLECTUAL PROPERTY OFFICE OF THE PEOPLE S REPUBLIC OF CHINA EXAMINATION DECISION OF INVALIDATION REQUEST
 PATENT REEXAMINATION BOARD OF THE STATE INTELLECTUAL PROPERTY OFFICE OF THE PEOPLE S REPUBLIC OF CHINA EXAMINATION DECISION OF INVALIDATION REQUEST Decision No. 9817 Decision Date April 29, 2007 Title
PATENT REEXAMINATION BOARD OF THE STATE INTELLECTUAL PROPERTY OFFICE OF THE PEOPLE S REPUBLIC OF CHINA EXAMINATION DECISION OF INVALIDATION REQUEST Decision No. 9817 Decision Date April 29, 2007 Title
HUNGARY Patent Act Act XXXIII of 1995 as consolidated on March 01, 2015
 HUNGARY Patent Act Act XXXIII of 1995 as consolidated on March 01, 2015 TABLE OF CONTENTS PART I INVENTIONS AND PATENTS Chapter I SUBJECT MATTER OF PATENT PROTECTION Article 1 Patentable inventions Article
HUNGARY Patent Act Act XXXIII of 1995 as consolidated on March 01, 2015 TABLE OF CONTENTS PART I INVENTIONS AND PATENTS Chapter I SUBJECT MATTER OF PATENT PROTECTION Article 1 Patentable inventions Article
Top Ten Tips for Dealing with Business Method Patents in Canada
 Top Ten Tips for Dealing with Business Method Patents in Canada Sep 01, 2011 Top Ten By Christopher Van Barr Grant Tisdall This resource is sponsored by: By Christopher Van Barr and Grant Tisdall, Gowling
Top Ten Tips for Dealing with Business Method Patents in Canada Sep 01, 2011 Top Ten By Christopher Van Barr Grant Tisdall This resource is sponsored by: By Christopher Van Barr and Grant Tisdall, Gowling
Part 1 Applications for Patents for Inventions
 Ministerial Regulations No. 21, B.E. 2542 (1999) Issued under the Patent Act B.E. 2522 Translation By virtue of the power granted under Sections 4, 17, 20, 33, 59 and 65 of the Patent Act B.E. 2522 and
Ministerial Regulations No. 21, B.E. 2542 (1999) Issued under the Patent Act B.E. 2522 Translation By virtue of the power granted under Sections 4, 17, 20, 33, 59 and 65 of the Patent Act B.E. 2522 and
Patentable Subject Matter and Medical Use Claims in the Pharmaceutical Sector
 Patentable Subject Matter and Medical Use Claims in the Pharmaceutical Sector 2012 LIDC Congress, Prague, 12 October 2012 Dr. Simon Holzer, Attorney-at-Law, Partner 3 October 2012 2 Introduction! Conflicting
Patentable Subject Matter and Medical Use Claims in the Pharmaceutical Sector 2012 LIDC Congress, Prague, 12 October 2012 Dr. Simon Holzer, Attorney-at-Law, Partner 3 October 2012 2 Introduction! Conflicting
Intellectual Property Reform In Australia
 Intellectual Property Reform In Australia January 2013 A summary of important legislative changes PATENTS TRADE MARKS DESIGNS PLANT BREEDER S RIGHTS Robust intellectual property rights delivered efficiently
Intellectual Property Reform In Australia January 2013 A summary of important legislative changes PATENTS TRADE MARKS DESIGNS PLANT BREEDER S RIGHTS Robust intellectual property rights delivered efficiently
INTELLECTUAL PROPERTY LAWS AMENDMENT (RAISING THE BAR ACT) 2012
 INTELLECTUAL PROPERTY LAWS AMENDMENT (RAISING THE BAR ACT) 2012 AUTHOR: MICHAEL CAINE - PARTNER, DAVIES COLLISON CAVE Michael is a fellow and council member of the Institute of Patent and Trade Mark Attorneys
INTELLECTUAL PROPERTY LAWS AMENDMENT (RAISING THE BAR ACT) 2012 AUTHOR: MICHAEL CAINE - PARTNER, DAVIES COLLISON CAVE Michael is a fellow and council member of the Institute of Patent and Trade Mark Attorneys
THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : INDIAN COMPANIES ACT, 1913 CS (OS) No. 563/2005 Date of Decision:
 THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : INDIAN COMPANIES ACT, 1913 CS (OS) No. 563/2005 Date of Decision: 22.03.2013 TATA SONS LTD. & ANR.....Plaintiff Through: Sh. Pravin Anand, Sh. Achutan Sreekumar,
THE HIGH COURT OF DELHI AT NEW DELHI SUBJECT : INDIAN COMPANIES ACT, 1913 CS (OS) No. 563/2005 Date of Decision: 22.03.2013 TATA SONS LTD. & ANR.....Plaintiff Through: Sh. Pravin Anand, Sh. Achutan Sreekumar,
