CHAPTER SEVEN INDIAN PATENT LAW AND ITS COMPATIBILITY TO. TRIPs UNDER WTO
|
|
|
- Alyson Sullivan
- 5 years ago
- Views:
Transcription
1 CHAPTER SEVEN INDIAN PATENT LAW AND ITS COMPATIBILITY TO TRIPs UNDER WTO The Patents Act 1970, along with the Patents Rules 1972, came into force on 20th April 1972, replacing the Indian Patents and Designs Act The first major transition in the Indian patents act 1970 which came into force on 20th April The Patents Act was largely based on the recommendations of the Ayyangar Committee Report headed by Justice N. Rajagopala Ayyangar2. One of the recommendations was the allowance of only process patents with regard to inventions relating to drugs, medicines, food and chemicals. Later, India became Signatory to many international arrangements with an objective of strengthening its patent law and coming in league with the modem world. One of the significant steps towards achieving this objective was becoming the member of the Trade Related Intellectual Property Rights (TRIPs) system. It is one of the controversial areas to amend the Patents Act to make it conform to these international agreements4. tushikand Hasija, Patent Pharmaceuticals: The Indian Scenario-.M.D.U.law journal. vol.9,2004,p , the Government of India appointed Justice N. Rajagopala Ayyangar Committee to examine the stion of revision of the Patent Law and advise government accordingly though the India was contracting parties of GATT jraham B.Paul.The Emersins Patents andiprs Resime Implications for Indian Industry,Globalization India,p
2 As TRIPs became a part of the WTO regime the member countries became bound to provide intellectual property protection as per TRIPs provision and were forced to amend their laws in tune with TRIPs.The TRIPs agreement have motivated the members countries to formulate more or less uniform IPR laws 5. WTO's dispute settlement mechanism6 made sure that those countries which failed to amend their laws forced to do so. For example, on a complaint by the US, the WTO appellate body held that India's patent law violated. (i) Article 70.8(a) of TRIPs7, and (ii) Article 70.9 of TRIPs8 As a result, India was forced to amend the patent law in 1999 with effect from The binding nature of the TRIPs Agreement is likely to have immense impact once all the member countries become bound to implement an across-the-board product patent regime. Before the Agreement came into force many developing countries, including India, allowed patents only for pharmaceutical processes and not for pharmaceutical products9. Some countries like Brazil, Thailand and Korea simply did not include medicines within the patent laws. Due to a weak patent regime generic versions of patented medicines could be produced locally and therefore, the local prices of a formulation were much lower compared to that in the developed world. However, a product patent results in a complete monopoly in favour of a patentee and he becomes free to manipulate the market price of the product. Arguably, patent rights for pharmaceutical products result in higher prices making the drugs 5 Ray Alok, Intellectual Property Rizhts in WTO Resime, The law of IPRs-Editor,S.S. Singh, Deep and Deep,p.61 6 See Rao M.B. WTO Dispute Settlement lexis Nexis Buuterworths Publications. 7 by failing to provide a means for the filing of patent applications for pharmaceuticals and agricultural chemical products 8 for not providing exclusive marketing rights (EMRs) to pharmaceutical and agricultural products. 9 See Indian Patent Act,
3 inaccessible for the poor. There, thus, seems to be an apparent conflict between the TRIPs regime and human rights values10. Under the World Trade Organisation obligations, each member-state is required to provide for a minimum level of protection of IPR embodied in the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPs). The recent changes in IPR laws reflect India's compliance with the obligation under the TRIPs Agreement. The TRIPs Agreement signed in 1994 has provided ten years time from to the countries who became its signatories for providing product patent protection to inventions which were not protected through product patents at the time of entering into the agreement vide Article 65(4) of the TRIPs Agreement. Since India became a member of WTO on in order to satisfy this requirement our Parliament amended the Patents Act so as to make it in conformity with the TRIPs Agreement. In order to comply with the obligations under the TRIPs agreement, as a WTO founding member, India in recent past undertook several structural amendments were introduced in the national patent law* 11. Our legislatures are called upon to amend our laws in conformity with the international treaties. For example, The Copyright Act, has been amended to include computer program as literary work as required by Article 10 of the TRIPs13 Agreement. The Trade and Merchandise Marks Act, has been replaced with the Trade Marks Act, 1999 which includes protection of well-known marks, certification marks and collective marks. It now provides for registration 10 Gupta Amit and Patel,Aditi, A Human Right approach to TRIPsA SCC (J) Kaushik, H.Hasiia.Patent Pharmaceuticals: The Indian Sce»ar/o.MDULJ.2004.p It came into force on TRIPs is known as Magna Carta of the IPRs 14 Repealed by TM Act,
4 of trade mark for services as well. This is in compliance with Article 16 of the TRIPs Agreement. Other recent legislations include the Geographical Indications of Goods (Registration and Protection) Act, 1999, The Designs Act15, 2000 and The Protection of Plant Varieties and Farmers' Rights Act, The Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPs Agreement) imposes several obligations on the Government of India in the area of patent protection. It calls for substantial changes in the Indian Patent Act, 1970 to satisfy these obligations. The Agreement helps India to introduce the changes at three different stages. To satisfy the first set of obligations, the Patent (Amendment) Act, 1999 was enacted after India lost the dispute with USA in WTO16. The Patent (Amendment) Act, 2002 was intended to satisfy the second set of obligations. This included changes in patentable inventions, grant of new rights, extension of the term of protection, provision for reversal of burden of proof in case of process patent infringement and conditions for compulsory licenses17. The Patent (Amendment) Act, 2005 make the Indian patent Act fully compatible18. The preceding fifteen years have seen many new IPR enactments. With globalization, liberalization and privatization, the ambit of IPR has grown multifold and its importance has amplified, having a profound impact on commercial interests19. The TRIPs agreement made drastic changes in the international patent regime. It prescribed a universal minimum protection for patent including the scope of patentability, rights of the patentee, duration of protection, 15 It was enacted with an objective to consolidate and amend the law relating to protection of Design 6 Under the WTO Dispute Resolution Meehan ism,india has lost its case related to Implementation of TRIPs provisions. 17 Gopalakrishnan N.S.The Patents (Second Amendment) Bill An Analysis. (2001)1SCC (Jour) 14 8 Venkatarammu Preeti.Protection of inventions: A bird eve of Patent Laws in JW/a.Intellectual Property ^aws-sreenivasulu N.S.,Regal Publications 19 Lahoti R.C.,Justice.Role of Judiciary in IPR development and adjudication, (2004) 8 SCC (J) 1 198
5 compulsory licensing, burden of proof and set-up an enforcement mechanism. The developing countries were given a transitional period to switch over to the new regime. However, this transitional benefit was neutralized by the obligation on the developing countries to provide exclusive marketing rights for agrochemicals and pharmaceuticals. Thus, the developed countries had to change their intellectual property laws, especially patent law, to make it in compliance with the TRIPS Agreement. Therefore, the crucial question is how the developing countries can make their laws TRIPS compatible and at the same time safeguard their core developmental objectives. B. The Main provisions of TRIPs Articles 3, 4, 5 and 70 of the TRIPs are of peculiar importance and deals within the national treatment20, Most, favoured nation21 and multilateral agreement on acquisition of IPRs. 1. Patent without discrimination, subject to the normal tests of novelty, inventiveness and industrial applicability The TRIPs Agreement required Member countries to make patents available for any inventions whether products or processes, in all fields of technology without discrimination, subject to the normal tests of novelty, inventiveness and industrial applicability23. There 0 Article 3 of the TRIPs agreement provides for the same treatment as nationals of countries of the nion.,for the nationals of countries outside the union who are domiciled or have real and effective onnection in the territory of one of the countries. 1 Article 4 of the TRIPs agreement providesthe protection of intellectual property,any dvantage,favour,privilege or immunity granted by a member to the nationals of any country shall be scorded immediately and unconditionally to the nationals of all other members.. 2 Article 5 of the TRIPs agreement deals with the obligationunder Article 3 and 4 do not apply to rocedures provided in multilateral agreemnts concluded under the auspices of the WIPO relating to the iquisition or maintenance of IPRs. Article 27.1.these are three litmus test for granting patent 199
6 are three permissible exceptions to the basic rule on patent ability. 2. Inventions contrary to order public or morality For inventions contrary to order public or morality; this explicitly includes inventions dangerous to human, animal or plant life or health or seriously prejudicial to the environment. The use of this exception is subject to the condition that the commercial exploitation of the invention must also be prevented and this prevention must be necessary for the protection of ordre public or morality24 3. Exceptions Members may exclude from patent ability diagnostic, therapeutic and surgical methods for the nr treatment of humans or animals 4. Plants and Animals-- Members may exclude plants and animals other than microorganisms and essentially biological processes for the production of plants or animals other than non-biological and microbiological processes. Moreover, the whole provision is subject to review four years after entry into force of the Agreement.Members may provide limited exceptions to the exclusive rights conferred by a patent, provided that such exceptions do not unreasonably conflict with a normal exploitation of the patent and do not unreasonably prejudice the legitimate interests of the patent owner, taking account of the legitimate interests of third parties 24 Article Article 27.3(a). 26 Article 27.3(B) 27 Article
7 5. Patent Period The term of protection available shall not end before the expiration of a period of 20 years counted from the filing date28 6. clear and complete Disclosure Members shall require that an applicant for a patent shall disclose the invention in a manner sufficiently clear and complete for the invention to be carried out by a person skilled in the art and may require the applicant to indicate the best mode for carrying out the invention known to the inventor at the filing date or, where priority is claimed, at the priority date of the application29.if the subject-matter of a patent is a process for obtaining a. product, the judicial authorities shall have the authority to order the defendant to prove that the process to obtain an identical product is different from the patented process, where certain conditions indicating a likelihood that the protected process was used are met30 7. Compulsory licensing and government use -- Compulsory licensing and government use without the authorization of the right holder are allowed, but are made subject to conditions aimed at protecting the legitimate interests of the right holder. The conditions are mainly contained in Article Article 33 Article 29.1 C. Difference between TRIPs,Provisions and Indian Laws The main provisions of TRIPs in so far as they are different from the existing form of protection under the Patents Act (1970) were:! Article 34 of TRIPs Agreement 201
8 1. Patent terms A minimum patent terms of 20 years from the date of filing,1 3 'Product 42 * 6 patent for pharmaceutical and agricultural yy chemical -products. 2. Working of patent Importation to be accepted as "working of patent"33 Compulsory licensing to be confined to special circumstance such as emergency or abuse of patent rights. (Article 31 of TRIPS Agreement.). 3. Burden of proof Reversal of the burden of proof in legal actions over process patents -this will oblige the defendant to prove that his process is non- infringing Micro organisms to be patented 5. Effective protection for plant varieties Effective protection for plant varieties either by patents or by an effective sui generis system. D. Obligation of India under TRIPs (a) To recognize in principle all kinds of inventions - India has under obligation to recognize in principle all kinds of inventions in the area of pharmaceutical and agricultural chemical products in accordance with Article 27 of the Agreement, (b) To provide a mechanism for filing applications- India has to set up new mechanism as prescribed by the TRIPs agreement by which applications can be filed for new inventions as understood in Article 27 in these areas from , 1 Article 33 of TRIPs Agreement. 2 Article 27of TRIPs Agreement 3 Article 28 of TRIPs Agreement. 4 Article 34 of TRIPs Agreement Aricle 27(2) of TRIPs agreemnt 6 Article27 b of TRIPs Agreement 202
9 (c) To apply the test of patentability - according to the TRIPs agreement India has to apply the test of patentability as laid down in the Agreement, irrespective of the law of the country on the date of filing, at the time when patent is granted or rejected, (d) To provide patent protection for a period of 20 years- Before 1995 there is different patent protection in India which is 14 years. But under TRIPs agreement it is uniform patent protection to every member country. So India should provide patent protection for a period of 20 years from the date of filing once the parties decide to grant patent, (e) Grant exclusive marketing rights for five years in the case of product patent applications in these areas, grant exclusive marketing rights for five years or until patent is granted or rejected whichever period is shorter. Granting of exclusive marketing rights is subjected to three conditions: (i) Product patent granted by another member country, Product patent for the invention has been granted by another member country, (ii) Market approval - Market approval is obtained in such other member country and (iii) Market approval from the country/member granting exclusive marketing right is granted. E. The Year 1995 On account of signing of the TRIPs Agreement from , the Indian Patents Office has started receiving applications for pharmaceutical substances as per Article 70 of GATT. These applications are commonly called Black Box applications and have been taken up for consideration only after for grant of patent. 203
10 This provision has been included to protect new inventions made after satisfying the requirements of Article 27 of the TRIPs Agreement. It is also intended to exclude this benefit to inventions made before That is the reason why the provisions clearly stated that the inventions must take place after and insisted for a patent grant from another member. If one were to examine the Indian Patents Act, 1970, Section 5 of the Act expressly prohibits product patents protection for inventions relating to medicines or drugs and substances prepared or produced by chemical processes, This in fact excludes product patent for some items of pharmaceutical products and agricultural chemical products as envisaged in the TRIPs Agreement. According to Article 27, patent shall be granted to inventions in all fields of technology both for product and process. This is obligatory if the invention is new, involves an inventive step and is capable of industrial application. It also mandates that the patent rights must be made available irrespective of the place of invention or field of technology, and whether the products are imported or locally produced. India signed the Uruguay round Agreement in 1995 as founding member. This led to a number of amendments in the Indian Legislations and formulation of economic Policies to implement the WTO agreement Through India had a transition period of 5 years (w.e.f ) under Art 65 to apply the provisions of the agreement and an additional period of 5 years for extending product patent protection to areas of technology no protected so far, certain obligations were required to be fulfilled w.e.f To satisfy the first set of obligations, the Patent (Amendment) Act, 1999 was enacted after India lost the dispute with USA in WTO. The Patent (Second Amendment) Bill, 1999 was intended to satisfy the second set of obligations. This included changes in patentable inventions, grant of new rights, extension of Mittal J,K WTO and India.. New Era Publication, (p.7) I Vikas T>v..Law and Practice of Intellectual Property, Bharat Publication at p
11 the term of protection, provision for reversal of burden of proof in case of process patent infringement and conditions for compulsory licences. India has amended in 2005 to introduce protection for pharmaceutical products, agricultural chemical products, inventions relating to atomic energy and plant varieties. Apart from the provisions for satisfying TRIPs obligations some other provisions are also included in the Bill to modernise the Indian Patent Act to suit the requirements of the technological changes 39. In order to comply with the obligations under the TRIPs agreement,as a WTO founding member, India in recent past undertook several structural amendments were introduced in the national patent law.40 E. Amendments of Indian Intellectual Property Legislations: To meet the above requirements India has initiated the process for the comprehensive amendment of its IP legislations. India has complied with its obligations under TRIPs through a series of amendments of its existing laws and enacting new legislations. They are; The Trade Marks Act, The copy right (Amendment) Act, The Designs Act, , The Protection of Plant Varieties and Farmer s Right Act, The Semi-conductor and Integrated Circuits Layout Designs Act Gopalakrishnan N S.The Patents (Second Amendment) Bill An Analysis (2001)1SCC (Jour)14 40kaushik,and Hasiia.Patent Pharmaceuticals: the Indian scewflr/o.m.d.u.law journal. vol.9,2004,p Supra note Supra note Supra note Same was notified in
12 The Geographical Indications of Goods (Registration and Protection) Act, 1999 The Patents (Amendment) Act, 1999 The Patents (Amendment) Act, 2002 The Patents (Amendment) Act, 2005 After conclusion of the Uruguay Round at Marrakesh (Morocco) which adopted WTO - TRIPs Agreement, the next step was to be the issue of implementation in most of the developing countries India after authenticating the final Act on 15th April 1994, promulgated an ordinance on 31st December 1994 amending the Patents Act, 1970 and acceding to WTO. This ordinance was the first step to comply with immediate obligations under Article and Article INDIAN PATENT (AMENDMENT) ACT, 1999 The Patents (Amendment) Act, 1999 specified four pre-conditions to be met by an EMR applicant: (a) The applicant must hold a valid patent on pharmaceutical product granted after January 1, 1995 in any of the WTO member countries (countries who are also WTO members); (b) The applicant, should have marketing rights in the member countries; (c) A product patent application should already have been made in India, and (d) Marketing approval of the same product should have been granted in India. The first three conditions were as per the stipulation of TRIPs agreement. The fourth clause was incorporated to meet the Indian drug regulatory approval. The EMRs were to be given for a period of 5 years as per TRIPs requirement. The other important change made was the removal of restriction on residents to apply for patents outside India. In the Patent Act (1970) it was obligatory for residents (section 39) to seek prior permission before applying for patents outside India. icceptance of applications for product patents for Pharmaceutical and agricultural chemical products m by providing a mailbox -rant of Exclusive Marketing Rights (EMRs) to these products for five years 206
13 As mentioned earlier the 1999 (Amendment) Act to the Patent Law deals with the minimal amendments, which were necessary to be done and from which there was no escape, after India lost the case against USA & EC in WTO Dispute Settlement Body47. The Patents (amendment) Act 1999 introduced two main changes to the Act. Firstly, it introduced a new sub-section to section 5 prohibiting product patents on medicines and drugs. The new clause left the prohibition untouched but permitted the filing of a patent claim. Secondly patent applications for food or health related products had to be dealt with according to a new Chapter IVA which set out the, specific conditions under which this was to take place and provided for the grant of exclusive marketing rights as called for under TRIPs Agreement.48 While the adoption of the 1999 amendments proved to be a lengthy process, it constituted a tiny part of the overall changes that had to be put in place of TRIPs compliance. Since India had to comply with the most of its other TRIPs obligations by 1 January 2000, this led to the introduction of another proposed set of amendments in December These amendments were referred to a Parliamentary Committee that studied the proposed changes for the better part of the next two years. Apart from the amendments made in March, 1999, the Patents Act, 1970 has not undergone any change till During this period of time, however, there has been considerable technological innovation and development of knowledge and the concept of IP as a resource for knowledge- based industries has become well recognised the world over. Development. of technological capability in India, coupled with the need for integrating the IP system with international practices and intellectual property regimes require that the Patents Act, 1970 be modified into a modern, harmonised and user-friendly Act to adequately protect national and public interests, while simultaneously meeting India s international obligations under the TRIPs Agreement. 47 See Rao M.B.and Guru Manjula WTO Dispute Settlement and developing Countries.Lexis Nexis,Legal tto Business, ,Patents (Amendment) Act 1999, Gazette of India,26 March,
14 Eventually, the amendments proposed by the government in 1999 were adopted without major changes in The Highlights of the Patents (Amendment) Act, are: The Patent (Second Amendment) Bill, 1999 was intended to satisfy the second set of obligations. This included changes in patentable inventions, grant of new rights, extension of the term of protection, provision for reversal of burden of proof in case of process patent infringement and conditions for compulsory licences. India has time up to 2005 to introduce protection for micro-organism, pharmaceutical products, agricultural chemical products, inventions relating to atomic energy and plant varieties. Apart from the provisions for satisfying TRIPs obligations some other provisions are also included in the Bill to modernise the Indian Patent Act to suit the requirements of the technological changes50 A) Product Claims allowed for medicine/drug & agrochemicals except for those ordinarily used as intermediates in the preparation/manufacture of such substances, under certain conditions, such as: i) Patent Applications made under the above provision shall be consigned/kept in a "Black Box" and shall not be examined till 31-st December However, in the interim period Exclusive Marketing Rights (EMR) for a period of 5 years may be given on applying specifically for EMR, subject to fulfilling certain conditions. ii) Term of EMR for a period of 5 years from the date of approval or till the date of grant of patent or till the date of rejection of application for the grant of patent, whichever is earlier. iii) Exclusion of substances based on system of Indian Medicine. K,aushik.and.Hasiia Patent pharmaceuticals: the Indian scenario-.m.d.u.law journal. 9,2004,p.281,VISWANATHAN, AJndia:Patent (Amendment) Actl999.International Business /yer, VOL.27,no.9,1999, ,see also Naraynan,P. Indian Patent Law,Eastern Book Company. Gopala Krishnan N.S.The Patents (SecondAmendment) Bill, 1999-An Analysis (2001)1 SCC(Jour)
15 iv) Compulsory License: after 2 years from date of approval of EMR. v) Importation deemed to be working of Patent. B) Residents allowed to apply for Patents outside India without first filing for Patent in India, as was the case in the Indian Patents Act 1970, Section 39 has been omitted in the Amended Act The Patents (Amendment) Act, 2002 India amended its Patents Act again in 2002 to meet with the second set of obligations (Term of Patent etc.), which had to be effected from The Patents (Amendment) Act, 2002 closely follows TRIPs and in the process does away with provisions of the 1970 Act that constituted India s own response to the challenge of providing exclusive commercial rights in a field concerned with the fulfilment of basic health needs51. India amended its Patents Act again in 2002 to meet with the second set of obligations (Term of Patent etc.), which had to be effected from This amendment, which provides for 20 years term for O the patent, Reversal of burden of proof etc. came into force on 20th May, The Third Amendment of the Patents Act 1970, by way of the Patents (Amendment) Ordinance 2004 came into force on 1st January, 2005 incorporating the provisions for granting product patent in all fields of Technology including chemicals, food, drugs & agrochemicals and this Ordinance is replaced by the Patents (Amendment) Act 2005 which is in force now having effect from Some of the salient features of the Patents (Amendment) Act amendments are as follows: 51 Cullet Philipp ^Amended Patents Act and Access to Medicines after Do/?a-EPW-vol.37.June 15-June 21, Kaushik,and Hasiia Patent Pharmaceuticals: the Indian ScenarioM.D.V.law journal. vol.9,2004,p
16 Patent rights and terms of patents: Under section 48 of the amended act, the patent owners will have the exclusive right to Prevent others who do not have their consent, not only from making, using or selling the invented product or process in India, but also importing from other countries. Under the original 1970 Act, importing was not mentioned as an exclusive right. This is however subject to the provision in Section 107A (b), inserted in the amended act, which permits parallel imports. Under this section, importation of patented products by any person from a person who is duly authorised by the patentee to sell or distribute the product, will not be considered to an infringement of the patent right. Thus if a patented product is offered for sale in another country at a lower price by the patent holder or with the patent holder s consent, the patent holder here cannot legally stop its imports by others. The term of the patent granted will be 20 years from the date of filing of the application for the patent53. Under the 1970 act, the term for pharmaceuticals, was five years from the date of sealing or seven years from the date of the patent, whichever is shorter. (For other products the term was 14 years). Section 47 of the original Patents Act of 1970 contains a research exemption for patented inventions54. This section, which can be interpreted as applicable for both academic and commercial research, has been left unmodified by all subsequent amendments to the patent regime. But three major changes introduced in the Amendments of 2002 affect the patenting of research tools for biomedical and biotechnological inventions in India. These are as follows: The Patent Act has extended the scope of patentable inventions to a method or process of testing during the process of manufacture, including those in biochemical, biotechnological and microbiological areas. section 53(1) >ee section 47(3) 210
17 Agreement. Further the amendments seek to provide as extensive as a scope for section 5, which restricts patentability to process, patents by specifying that the chemical processes referred to include biochemical, biotechnological and microbiological processes. A special mention may be made of the question of the protection of the plant varieties. The revised section 3(j) specifically rejects the patentability of seeds and plant varieties. However article 27(3) (b) requires protection of plant varieties. One of the few areas where government can choose the protection system they want through the sui- generis option. In this case, the government decided to use this option and chose to draft a separate Act for this purpose. The Plant Variety Protection Act, which introduces Plant Breeder s Right and farmer s rights. The amendments also seek to provide answers to some of the new issues facing the patent system. The amendments provide at least a partial answer to biopiracy concerns by requiring the disclosure of the sources and geographical origin of the biological material used in chemical inventions. This is supplemented by a provision, which makes the failure to disclose the source and geographical origin of the biological material used a ground for opposing the grant of patents. This act also indirectly addresses questions related to the traditional knowledge protection by denying the patentability of traditional knowledge. Thus the act now allows the generic producers to get ready for marketing their products immediately after the patents expire, thereby reducing the time lag between patent expiration and availability of generics58. The system of compulsory licensing was strengthened. Interestingly, section 83 specifically mention that the patents granted should not hamper the protection of the public health and should not prohibit the Central Government from taking measures to protect the public health. The patents should be granted to make the 58 Chaudhury Sudip, TRIPS Agreement and amendments of the Patents Act in India. 37/32, Economic and Political Weekly (10 August 2002)p.3354.The first such provision was introduced in the United States in a revision of Federal Food, Drug and cosmetic act, PL98-417(1984)at section
18 0 Section 3 of the Patent Act that deals with inventions that are not patentable was amended in 2002 to include any process for the diagnostic or therapeutic treatment of human beings or for a similar treatment of animals or plants55 As a result of these provisions, biomedical research tools are patentable under Indian patent law. Medical, diagnostic and therapeutic kits/ tools are not patentable only when they are for the treatment of human beings or animals or plants. The Patents (Amendment) Act, 2002 closely follows TRIPs and in the process does away with provisions of the 1970 Act that constituted India s own response to the challenge of providing exclusive commercial rights in a field concerned with the fulfilment of basic health needs56. The amendments adopted in 2O0257 have removed most of the elements that gave the Patents Act The most important impact of this change was the balance between the interests of the patent holders and the society s interests. Some of the changes include the increase of the term of protection to a uniform 20-year term, thereby increasing significantly the average duration of protection and removing the discrimination put in the place in the case of process patents in the field of health and nutrition where the term was only for 7 years. In the midst of changes which significantly reinforce the position of patent holders, the amendments also seek in some respect to limit the rights of the patent holders. The specific flexibility offered by the TRIPs Agreement has been used in several cases. The environmental and health exceptions were authorized by Article 27(2) of the TRIPs Agreement are drafted into the section 3(b).Similarly a new section 3(j) used all exceptions allowed under Article 27(3) of TRIPs 55 Section 3(i)). 56 Philippe Cullet Amended Patents Act and Access to Medicines after Doha,EPW June 15 - June 21, This Act makes the Indian patent law not only TRIPs compliant but also incorporates safeguards for the protection of public interest, national security, bio-diversity, traditional knowledge, etc. The opportunity has also been utilised to harmonise the patent granting procedures with international practices and to make the system user friendly. 211
19 benefits of the patented inventions available at a reasonably affordable price to the public. The 2002 amendments was followed by a set of amendments which are required to put India in compliance with its obligations to introduce the product patents in health and food sector. A Bill 2003 was introduced at the end of 2003.The Bill was not passed by the parliament the government promulgated a temporary ordinance after the end of the parliamentary session which had the effect of putting the country in compliance with its TRIPs obligations. The Patents (Amendment) Act, 2002 was passed by Parliament in May, 2002 and notified in June, The Act has been made effective from May, 2003 and has brought about lot of changes. Modification of term invention59: The Sec. 2 (l)(j) of Patent (Amendment) Act 2002, defines the term invention as "a new product or process involving an inventive step and capable of industrial * O application" Where Inventive step means a feature that makes the invention not obvious to person skilled in the art. Earlier invention means any new and useful - (i) Art, process, method or manner of manufacture; (ii) Machine, apparatus or other article; (iii) Substance produced by manufacture; and includes any new and useful improvement of any of them, and an alleged invention. Examination of application60 59 See section 2(1 )j 213
20 India has opted for a deferred examination system. This means the Controller will not initiate examination of the application. Examination of an application will now be taken up only upon request by applicant or in the Form 19 with fees of Rs.1000 for individual applicant or Rs for legal entity other than an individual, at the appropriate office of the Patent office (Rule. 24). The request is to be made within forty-eight months from the application filing date. Where an application was filed prior to May 20, 2003 (i.e. before the commencement of the Patent (Amendment) Act 2002), a request for examination is required to be made within a period of twelve months from May 20, 2003 or forty-eight months from the filing date, whichever is later. Upon failure to request examination, the application shall be treated as withdrawn by the applicant. The applicant or agents can also withdraw the application at any time (before the grant of the patent) after filing the application. Ptiblication/Notification of the Application61: After the expiry of 18 months from the date of filing or the date of priority whichever is earlier, the Controller will notify the contents of the applications falling within the provision in the Gazette of India Part-Ill Sec.2 (Rule 25). Term of Patent62: The term of patent has been enlarged to twenty years for existing patents and patents granted on pending applications. This term is calculated from the date of filing of the application. Earlier the term of patent for method or process of manufacture of substance (e.g. food, medicines, drugs etc.) was five years from the date of the sealing of the patent, or seven years from the date of patent whichever period is shorter and in respect of any other invention, fourteen years from the date of the patent. i Sec. 11(b) Sec. 11 (a) : Sec
21 Burden off proof63: The burden of proof in a proceeding for process patent infringement has been reversed and imposed on Defendant. Prohibition to apply abroad 64: No person shall file an application or patent for an invention without applying in India or without the written permission of the Central Govt. If the applicant is not interested to secure a patent in India or the invention is not patentable according to the Indian law, he has to mandatory file an application for the said invention and has to wait for the expiry of six weeks after filing the application and then only file the corresponding application abroad for the same invention. Date of Patent65: The date of every patent will be the date of filing the application for patent. According to The Patent Act 1970, the date of patent was the date of filing of complete specification. The date of patent is very important to determine the term of parent. Rights of patentee66: The rights can be consider as negative because the rights of patentee, in the case of product patent, prevent third parties without the consent of the patentee, from making, using, offering for sale, selling or importing into India and the rights in the case of process patent, prevent third parties without the consent of the patentee, from the act of using that process and offering, for sale or selling in India or importing for those purposes the product obtain directly by that process, provided that the product obtained is not patentable under the Act. 63 Sec. 104 A 64 Sec Sec Sec
22 The Powers of the Branch Office : The branch offices of the Patent office have been vested with more powers. Under Sec. 68 the actions such as making a request of sealing of patents, registration of assignment 68etc has to be made in the appropriate Branch offices of the Patent office and not at the Head Office as the case earlier. Appellate Board69: Appeal Board appointed under the Trade and Merchandise Marks Act 1999 shall be the Appeal Board for purposes of Patents Act. This Appellate Board hears and decides appeals from the decision of the controller. The Head Quarter of the Appeal Board is to be in Chennai. It is clear that the definition of term invention in Patent Act, 2002 has enlarged the scope of protection. The controller has also been vested with the power to consider the question of obviousness of the invention disclosed while conducting the examination of application for considering the grant of a patent for the invention. In this context it should be noted that in the Patent Act, 1970 the Controller has no direct power to consider the question of obviousness. The power is only for the opponents while opposing the draft of patents under Sec. 25 of the Patent Act, The effect of this amendment is that it may not be possible to get a patent for trivial modifications. Time for placing the application in order for acceptance70: The time frame for putting an application in order for acceptance subsequent to its first examination has been shortened to 12 months (non-extendible) from the date of First Examination Report (FER). The first reply to the first examination report is required to be made within 4 months of the date of its issuance. Rule 4 Sec. 68 Sec. 2 (l)(a) Sec
23 Unity of Invention71 : The concept of 'unity of invention' has been broadened to include a group of inventions linked so as to form a single inventive concept. The claims in a specification should relate to a single invention or a group of invention linked so as to form a single inventive concept. Now, by this amendment it may be possible to claim more than one process in a single application if these processes fall under one group and are closely linked. Electronic Communication72: The documents can also be filed by electronic transmission duly authenticated. In that event the document should be clear, properly addressed and mailed and its original has to be submitted within fifteen days from the date of receipt of communication. The drawings can also be filed electronically. 7? Statement and Undertaking : For filing the Statement and Undertaking on Form 3 a provision has been provided to seek extension beyond three months. Declaration of inventor-ship74 The declaration of inventor ship on Form 5 should be filed along with the complete specification. An extension of one month beyond this period can be secured by filing a request on Form 4 with the fees Rs. 250/- pm if the applicant is an individual or Rs. 1000/- pm if the applicant is a legal entity. Abstract75: 71 Sec. 10(5) 72 Rule 6 73 Rule 12(4) 74 Rule 13 (6) 75 Rule 13 (a) to (d) 217
24 While filing the application accompanied with a complete specification, an abstract of the invention maximum 150 words have to be filed. Application76: An application for patent corresponding to International application (PCT application) has to be filed on Form 1 A. Licenses of right77: The Provisions relating to "licenses of right" deleted. Restoration of lapsed patent78 : The time for filing the request for restoration of the lapsed patent has been extended from one year to eighteen months. The 2002 Amendment to the Indian Patents Act had provided for Patenting of microorganisms and microbiological processes - thus opening the door for patent «o protection to biotechnological inventions both in the agricultural sector (genetically modified seeds, etc) and in the case of pharmaceuticals produced through biotechnology. This provision which follows the TRIPs provisions of the same kind, was one of the most controversial in the TRIPs agreement. As a result, at the time of signing of the agreement it had been agreed upon that this clause would be reviewed within 4 years. The clause is under review in the TRIPs council, but because no consensus has been arrived at, it continues to be a part of the TRIPs agreement. While this clause has not been deleted from the Indian Act,. the Govt, has given a commitment that the matter would be referred to a committee, and if felt necessary by the committee, the Act would be modified at a later stage. Rule 20(1) Sec. 86 to 88 Sec
25 Similarly, the Government has given a commitment that the issue of further defining what a pharmaceutical substance is to restrict the scope of patenting frivolous claims would be referred to a committee and further amendments can be considered based on the committee's findings. Indian Patents Act, 1970, amendment 2002, has excluded from patentability under Section 3(j), plants and animals as a whole or any part thereof other than microorganisms but including seeds, varieties and species and essentially biological processes for production or propagation of plants and animals and Section 3(i) 'any process for medical, surgical, curative, prophylactic (diagnostic, therapeutic), or other treatment of human beings, or any process for a similar treatment of animals to render them free of disease or to increase their economic value or that of their products79, The making of human body parts is not viewed as invention since they exist in nature, but modified or isolated body parts are viewed as multicellular organisms and treated as such for patentability if they meet the statutory requirements80. Patent (Amendment) Act 2005 The Third Amendment of the Patents Act 1970, by way of the Patents (Amendment) Ordinance 2004 came into force on 1st January, 2005 incorporating the provisions for granting product patent in all fields of Technology including chemicals, food, drugs & agrochemicals and this Ordinance is replaced by the Patents (Amendment) Act 2005 which is in force now having effect from 1-1- r9 This is in pursuance to the TRIPs Agreement Article 27.3 (a) and (b). Further TRIPS Article 27.2 nentions that States may exclude from patentability inventions, whose commercial exploitation within their erritory needs to be prevented to protect ordre public or morality including to protect human, animal or )lant life or health or to avoid serious prejudice to the environment'!0 K. V. Swaminathan, Patenting in Biotechnology. In Proc. Roving Seminar on 1PR in Biotechnology. CIMAP, Lucknow,
26 2005. Changes have been made in Patent Act 1970 by the patent (Amendment) Act, 2005 to meet the requirements of the TRIPs and PCT81. India was required to introduce product patent protection in these sectors from in accordance with the obligation under the TRIPs Agreement of the WTO. The Patents (Amendment) Act, 2005 introduces product patent regime for food, chemicals and pharmaceuticals. To fulfill this requirement, Government of India had issued an Ordinance in The Ordinance was to be approved by the Parliament. While introducing the Patents (Amendment) Bill 2005 in the Parliament, Government introduced certain O') changes from the provisions in the Ordinance. Features of the Patents (Amendment) Act, 2005 The main important changes which have been made in Patent Act 1970 are The Patent (Amendments) Act of 2005 has also extended the grounds on which a patent can be opposed in the pre-grant period. These are now contained in Section 25 of the 2005 Act. The earlier Ordinance had come under criticism on this point, since it reduced the grounds for pre-grant opposition of patents to only two (from the nine grounds listed in the original Act of 1970). The 2005 Act has now retained the nine grounds listed in the original Act of 1970 (with some modifications) and also added two more grounds of pre-grant opposition: on the lack of disclosure or no disclosure of source of geographical origin of biological material and another on the presence of indigenous knowledge, oral or written, of O'! local and indigenous communities in India m the invention. 1 Hands on Guide to Patents Act with patent /?tt/&s-taxmann s.p.i-5 2 The patent (Third) Amendment Act also provide for deletion of provisions relating to exclusive larketing right, which would become redundant, and steamlines the system by having both pre-grant and ost grant opposition to patents in pursuance of the TRIPs agreement negotiated during the Uruguay tound. 1 See section 25(j) and (k) 220
27 a) Extension of product patent protection to all fields of technology84; The Third Amendment Act, 2005 has introduce product patent protection in all fields of technology as envisaged in Article 27.1 b) Deletion of the provisions relating to Exclusive Marketing Rights (EMRs) (which would now become redundant), and introduction of a transitional provision for safeguarding EMRs already granted; c) Introduction of a provision for enabling grant of compulsory licence for export of medicines to countries which have insufficient or no manufacturing capacity, to meet emergent public health situations85 d) Modification in the provisions relating to opposition procedures with a view to streamlining the system by having both Pre-grant and Post-grant opposition in the Patent Office; e) Addition of a new proviso in respect of mailbox applications so that patent rights in respect of the mailbox shall be available only from the date of grant of patent, and not retrospectively from the date of publication. f) Strengthening the provisions relating to national security to guard against patenting abroad of dual use technologies; g) Rationalisation of provisions relating to time-lines with a view to introducing flexibility and reducing the processing time for patent applications, and simplifying and rationalising procedures. 84 i.e., drugs, foods and chemicals 85 In accordance with the Doha declaration on TRIPs and Public health 221
28 The Act has the effect of invalidating Section 5 of the Indian Patent Act, which granted only process patents for food, medicines and other drug substances. As a result, reverse engineering possibilities available to the pharmaceutical industry will only be limited to those drugs that are off-patent. The Act also introduces Section 92(A) on compulsory licensing, in keeping with 30 August 2003 Decision of the WTO. Section 92 (A) of the Act deals with compulsory licensing of pharmaceuticals for export purposes. This is meant to facilitate the Indian industry to continue supplying cheaper generic versions of patented drugs to those LDCs that do not have adequate domestic manufacturing capabilities. The Patent (Amendments) Act of 2005 was preceded by an earlier 2004 Patent (Amendments) Ordinance that was different in several aspects from the Amendments Act of 2005 that has now been enacted. For example, the 2004 Ordinance provided for exclusive marketing rights (hereafter, EMRs) that were to be effective under the same terms under which they were granted, and also laid out the power of the government to sell or distribute the article for which the EMR was granted and to direct that the EMR-based product be sold at a regulated price86. Expansion of the Scope of Patentability: Some of the key issues relating to the scope of patentability are given below. Inventive Step: The Bill.provides the following definition of what is required of a patent application to meet the inventive step criteria: a feature of an invention that involves technical advance as compared to the existing knowledge or having economic significance or both that makes the invention not obvious to a person skilled in the art. The above provision arguably broadens the existing provision to the benefit of patent holders and is ambiguous to the extent that it allows for two criteria for meeting an inventive step. As it stands, to meet an inventive step criteria the action 24(d),the patent amendment act 2005 now omitted section
Rksassociate Advocates & Legal Consultants ebook
 Rksassociate Advocates & Legal Consultants ebook Contents PATENTS 1. Types of Patent Applications 2. Patentable Inventions 3. Non-Patentable Inventions 4. Persons Entitled to apply for Patent 5. Check-List
Rksassociate Advocates & Legal Consultants ebook Contents PATENTS 1. Types of Patent Applications 2. Patentable Inventions 3. Non-Patentable Inventions 4. Persons Entitled to apply for Patent 5. Check-List
THE PATENT LAW 1. GENERAL PROVISIONS. Article 1. This Law shall regulate the legal protection of inventions by means of patents.
 THE PATENT LAW 1. GENERAL PROVISIONS Article 1 This Law shall regulate the legal protection of inventions by means of patents. Article 2 This Law shall also apply to the sea and submarine areas adjacent
THE PATENT LAW 1. GENERAL PROVISIONS Article 1 This Law shall regulate the legal protection of inventions by means of patents. Article 2 This Law shall also apply to the sea and submarine areas adjacent
BE it enacted by Parliament in the Fifty-sixth Year of the Republic of India as follows:-
 ~ THE PATENTS (AMENDMENT) ACT, 2005 # NO. 15 OF 2005 $ [4th April, 2005] + An Act further to amend the Patents Act, 1970. BE it enacted by Parliament in the Fifty-sixth Year of the Republic of India as
~ THE PATENTS (AMENDMENT) ACT, 2005 # NO. 15 OF 2005 $ [4th April, 2005] + An Act further to amend the Patents Act, 1970. BE it enacted by Parliament in the Fifty-sixth Year of the Republic of India as
The Patents (Amendment) Act,
 !"# The Patents (Amendment) Act, 2005 1 [NO. 15 OF 2005] CONTENTS [April 4, 2005] Sections Sections 1. Short title and commencement 40. Amendment of Section 57 2. Amendment of Section 2 41. Substitution
!"# The Patents (Amendment) Act, 2005 1 [NO. 15 OF 2005] CONTENTS [April 4, 2005] Sections Sections 1. Short title and commencement 40. Amendment of Section 57 2. Amendment of Section 2 41. Substitution
THE ACTS ON AMENDMENTS TO THE PATENT ACT */**/***/****/*****/******/*******
 Patent Act And THE ACTS ON AMENDMENTS TO THE PATENT ACT */**/***/****/*****/******/******* NN 173/2003, in force from January 1, 2004 *NN 87/2005, in force from July 18, 2005 **NN 76/2007, in force from
Patent Act And THE ACTS ON AMENDMENTS TO THE PATENT ACT */**/***/****/*****/******/******* NN 173/2003, in force from January 1, 2004 *NN 87/2005, in force from July 18, 2005 **NN 76/2007, in force from
PATENT ACT (UNOFFICIAL CLEAR TEXT) I. GENERAL PROVISIONS
 PATENT ACT NN 173/03, 31.10.2003. (in force from January 1, 2004) *NN 87/05, 18.07.2005. (in force from July 18, 2005) **NN 76/07, 23.07.2007. (in force from July 31, 2007) ***NN 30/09, 09.03.2009. (in
PATENT ACT NN 173/03, 31.10.2003. (in force from January 1, 2004) *NN 87/05, 18.07.2005. (in force from July 18, 2005) **NN 76/07, 23.07.2007. (in force from July 31, 2007) ***NN 30/09, 09.03.2009. (in
[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights
![[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights [English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights](/thumbs/90/103688579.jpg) Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Chile... Office: National Institute of Industrial Property (INAPI)...
Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Chile... Office: National Institute of Industrial Property (INAPI)...
LATVIA Patent Law adopted on 15 February 2007, with the changes of December 15, 2011
 LATVIA Patent Law adopted on 15 February 2007, with the changes of December 15, 2011 TABLE OF CONTENTS Chapter I General Provisions Section 1. Terms used in this Law Section 2. Purpose of this Law Section
LATVIA Patent Law adopted on 15 February 2007, with the changes of December 15, 2011 TABLE OF CONTENTS Chapter I General Provisions Section 1. Terms used in this Law Section 2. Purpose of this Law Section
FINLAND Patents Act No. 550 of December 15, 1967 as last amended by Act No. 101/2013 of January 31, 2013 Enter into force on 1 September 2013
 FINLAND Patents Act No. 550 of December 15, 1967 as last amended by Act No. 101/2013 of January 31, 2013 Enter into force on 1 September 2013 TABLE OF CONTENTS CHAPTER 1 General Provisions Section 1 Section
FINLAND Patents Act No. 550 of December 15, 1967 as last amended by Act No. 101/2013 of January 31, 2013 Enter into force on 1 September 2013 TABLE OF CONTENTS CHAPTER 1 General Provisions Section 1 Section
FINAL PROPOSAL OF THE ACT ON AMENDMENTS TO THE PATENT ACT
 FINAL PROPOSAL OF THE ACT ON AMENDMENTS TO THE PATENT ACT In the Patent Act ( Official Gazette Nos. 173/2003, 87/2005, 76/2007, 30/2009, 128/10 and 49/2011), after Article 1, Articles 1.a and 1.b are added
FINAL PROPOSAL OF THE ACT ON AMENDMENTS TO THE PATENT ACT In the Patent Act ( Official Gazette Nos. 173/2003, 87/2005, 76/2007, 30/2009, 128/10 and 49/2011), after Article 1, Articles 1.a and 1.b are added
ANNEX XVII REFERRED TO IN ARTICLE 5 PROTECTION OF INTELLECTUAL PROPERTY
 ANNEX XVII REFERRED TO IN ARTICLE 5 PROTECTION OF INTELLECTUAL PROPERTY ANNEX XVII REFERRED TO IN ARTICLE 5 PROTECTION OF INTELLECTUAL PROPERTY SECTION I GENERAL PROVISIONS Article 1 Definition of Intellectual
ANNEX XVII REFERRED TO IN ARTICLE 5 PROTECTION OF INTELLECTUAL PROPERTY ANNEX XVII REFERRED TO IN ARTICLE 5 PROTECTION OF INTELLECTUAL PROPERTY SECTION I GENERAL PROVISIONS Article 1 Definition of Intellectual
HUNGARY Patent Act Act XXXIII of 1995 as consolidated on March 01, 2015
 HUNGARY Patent Act Act XXXIII of 1995 as consolidated on March 01, 2015 TABLE OF CONTENTS PART I INVENTIONS AND PATENTS Chapter I SUBJECT MATTER OF PATENT PROTECTION Article 1 Patentable inventions Article
HUNGARY Patent Act Act XXXIII of 1995 as consolidated on March 01, 2015 TABLE OF CONTENTS PART I INVENTIONS AND PATENTS Chapter I SUBJECT MATTER OF PATENT PROTECTION Article 1 Patentable inventions Article
India Patent Act, 2003 Updated till March 11th, 2015
 India Patent Act, 2003 Updated till March 11th, 2015 TABLE OF CONTENTS CHAPTER I PRELIMINARY 1. Short title, extent and commencement. 2. Definitions and interpretation. CHAPTER II INVENTIONS NOT PATENTABLE
India Patent Act, 2003 Updated till March 11th, 2015 TABLE OF CONTENTS CHAPTER I PRELIMINARY 1. Short title, extent and commencement. 2. Definitions and interpretation. CHAPTER II INVENTIONS NOT PATENTABLE
THE PATENTS ACT 1970
 THE PATENTS ACT 1970 (39 of 1970) An Act to amend and consolidate the law relating to patents. (19 th September, 1970) Be it enacted by Parliament in the twenty first year of the Republic of India as follows;-
THE PATENTS ACT 1970 (39 of 1970) An Act to amend and consolidate the law relating to patents. (19 th September, 1970) Be it enacted by Parliament in the twenty first year of the Republic of India as follows;-
AUSTRALIA Patents Act 1990 Compilation date: 24 February 2017 Includes amendments up to: Act No. 61, 2016 Registered: 27 February 2017
 AUSTRALIA Patents Act 1990 Compilation date: 24 February 2017 Includes amendments up to: Act No. 61, 2016 Registered: 27 February 2017 TABLE OF CONTENTS Chapter 1. Introductory 1 Short title 2 Commencement
AUSTRALIA Patents Act 1990 Compilation date: 24 February 2017 Includes amendments up to: Act No. 61, 2016 Registered: 27 February 2017 TABLE OF CONTENTS Chapter 1. Introductory 1 Short title 2 Commencement
SWEDEN PATENTS ACT No.837 of 1967 in the version in force from July 1, 2014
 SWEDEN PATENTS ACT No.837 of 1967 in the version in force from July 1, 2014 TABLE OF CONTENTS Chapter 1. General Provisions Article 1 Article 1a Article 1b Article 1c Article 1d Article 2 Article 3 Article
SWEDEN PATENTS ACT No.837 of 1967 in the version in force from July 1, 2014 TABLE OF CONTENTS Chapter 1. General Provisions Article 1 Article 1a Article 1b Article 1c Article 1d Article 2 Article 3 Article
ETHIOPIA A PROCLAMATION CONCERNING INVENTIONS, MINOR INVENTIONS AND INDUSTRIAL DESIGNS PROCLAMATION NO. 123/1995 ENTRY INTO FORCE: May 10, 1995
 ETHIOPIA A PROCLAMATION CONCERNING INVENTIONS, MINOR INVENTIONS AND INDUSTRIAL DESIGNS PROCLAMATION NO. 123/1995 ENTRY INTO FORCE: May 10, 1995 TABLE OF CONTENTS CHAPTER ONE General Provisions 1. Short
ETHIOPIA A PROCLAMATION CONCERNING INVENTIONS, MINOR INVENTIONS AND INDUSTRIAL DESIGNS PROCLAMATION NO. 123/1995 ENTRY INTO FORCE: May 10, 1995 TABLE OF CONTENTS CHAPTER ONE General Provisions 1. Short
Compilation date: 24 February Includes amendments up to: Act No. 61, Registered: 27 February 2017
 Patents Act 1990 No. 83, 1990 Compilation No. 41 Compilation date: 24 February 2017 Includes amendments up to: Act No. 61, 2016 Registered: 27 February 2017 This compilation includes commenced amendments
Patents Act 1990 No. 83, 1990 Compilation No. 41 Compilation date: 24 February 2017 Includes amendments up to: Act No. 61, 2016 Registered: 27 February 2017 This compilation includes commenced amendments
INDUSTRIAL PROPERTY ACT, No. 8 of 2010 ARRANGEMENT OF SECTIONS. PART II Patents
 A.17 INDUSTRIAL PROPERTY ACT, 2010 No. 8 of 2010 ARRANGEMENT OF SECTIONS SECTION PART I Preliminary 1. Short title and commencement 2. Interpretation 3. Continuance of Marks, Patents and Designs Office
A.17 INDUSTRIAL PROPERTY ACT, 2010 No. 8 of 2010 ARRANGEMENT OF SECTIONS SECTION PART I Preliminary 1. Short title and commencement 2. Interpretation 3. Continuance of Marks, Patents and Designs Office
FINAL REPORT THE PATENTS AND DESIGNS ACT, INTRODUCTION PATENTS
 FINAL REPORT ON THE PATENTS AND DESIGNS ACT, 200----- INTRODUCTION PATENTS In England grants of monopoly rights to exploit an invention by the inventor date back to the Elizabethan (Queen Elizabeth I)
FINAL REPORT ON THE PATENTS AND DESIGNS ACT, 200----- INTRODUCTION PATENTS In England grants of monopoly rights to exploit an invention by the inventor date back to the Elizabethan (Queen Elizabeth I)
Questionnaire on Exceptions and Limitations to Patent Rights
 Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Office: India The Patent Office Person to be contacted: Name: Dr
Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Office: India The Patent Office Person to be contacted: Name: Dr
DECISION 486 Common Intellectual Property Regime (Non official translation)
 DECISION 486 Common Intellectual Property Regime (Non official translation) THE COMMISSION OF THE ANDEAN COMMUNITY, HAVING SEEN: Article 27 of the Cartagena Agreement and Commission Decision 344; DECIDES:
DECISION 486 Common Intellectual Property Regime (Non official translation) THE COMMISSION OF THE ANDEAN COMMUNITY, HAVING SEEN: Article 27 of the Cartagena Agreement and Commission Decision 344; DECIDES:
OFFICIAL GAZETTE OF THE REPUBLIC OF KOSOVA / No. 12 / 29 AVGUST 2011, PRISTINA. LAW No. 04/L-029 ON PATENTS LAW ON PATENTS
 OFFICIAL GAZETTE OF THE REPUBLIC OF KOSOVA / No. 12 / 29 AVGUST 2011, PRISTINA LAW No. 04/L-029 ON PATENTS Assembly of Republic of Kosovo; Based on Article 65 (1) of the Constitution of the Republic of
OFFICIAL GAZETTE OF THE REPUBLIC OF KOSOVA / No. 12 / 29 AVGUST 2011, PRISTINA LAW No. 04/L-029 ON PATENTS Assembly of Republic of Kosovo; Based on Article 65 (1) of the Constitution of the Republic of
The Patents Act 1977 (as amended)
 The Patents Act 1977 (as amended) An unofficial consolidation produced by Patents Legal Section 17 December 2007 UK Intellectual Property Office is an operating name of the Patent Office 1 Note to users
The Patents Act 1977 (as amended) An unofficial consolidation produced by Patents Legal Section 17 December 2007 UK Intellectual Property Office is an operating name of the Patent Office 1 Note to users
THE PATENT LAW 1 I INTRODUCTORY PROVISIONS. 1. Subject Matter of Regulation and Definitions. Subject Matter of Regulation.
 THE PATENT LAW 1 I INTRODUCTORY PROVISIONS 1. Subject Matter of Regulation and Definitions Subject Matter of Regulation Article 1 This Law shall regulate the legal protection of inventions. The invention
THE PATENT LAW 1 I INTRODUCTORY PROVISIONS 1. Subject Matter of Regulation and Definitions Subject Matter of Regulation Article 1 This Law shall regulate the legal protection of inventions. The invention
MODULE. Conclusion. ESTIMATED TIME: 3 hours
 MODULE 11 Conclusion ESTIMATED TIME: 3 hours 1 Overview I. MODULE 1 INTRODUCTION TO THE WTO SUMMARY... 3 II. MODULE 2 INTRODUCTION TO THE TRIPS AGREEMENT SUMMARY... 5 III. MODULE 3 COPYRIGHT AND RELATED
MODULE 11 Conclusion ESTIMATED TIME: 3 hours 1 Overview I. MODULE 1 INTRODUCTION TO THE WTO SUMMARY... 3 II. MODULE 2 INTRODUCTION TO THE TRIPS AGREEMENT SUMMARY... 5 III. MODULE 3 COPYRIGHT AND RELATED
Intellectual Property Laws Amendment Bill 2013 No., 2013
 00-0-0-0 The Parliament of the Commonwealth of Australia HOUSE OF REPRESENTATIVES Presented and read a first time Intellectual Property Laws Amendment Bill 0 No., 0 (Industry, Innovation, Climate Change,
00-0-0-0 The Parliament of the Commonwealth of Australia HOUSE OF REPRESENTATIVES Presented and read a first time Intellectual Property Laws Amendment Bill 0 No., 0 (Industry, Innovation, Climate Change,
GENEVA STANDING COMMITTEE ON THE LAW OF PATENTS. Thirteenth Session Geneva, March 23 to 27, 2009
 E WIPO SCP/13/3. ORIGINAL: English DATE: February 4, 2009 WORLD INTELLECTUAL PROPERT Y O RGANI ZATION GENEVA STANDING COMMITTEE ON THE LAW OF PATENTS Thirteenth Session Geneva, March 23 to 27, 2009 EXCLUSIONS
E WIPO SCP/13/3. ORIGINAL: English DATE: February 4, 2009 WORLD INTELLECTUAL PROPERT Y O RGANI ZATION GENEVA STANDING COMMITTEE ON THE LAW OF PATENTS Thirteenth Session Geneva, March 23 to 27, 2009 EXCLUSIONS
The methods and procedures described must be directly applicable to production.
 National Patent Administration Argentina Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 3 Section 3: Experimental use and/or scientific research... 3 Section 4: Preparation
National Patent Administration Argentina Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 3 Section 3: Experimental use and/or scientific research... 3 Section 4: Preparation
Courtesy translation provided by WIPO, 2012
 REPUBLIC OF DJIBOUTI UNITY EQUALITY PEACE ********* PRESIDENCY OF THE REPUBLIC LAW No. 50/AN/09/6 L On the Protection of Industrial Property Courtesy translation provided by WIPO, 2012 THE NATIONAL ASSEMBLY
REPUBLIC OF DJIBOUTI UNITY EQUALITY PEACE ********* PRESIDENCY OF THE REPUBLIC LAW No. 50/AN/09/6 L On the Protection of Industrial Property Courtesy translation provided by WIPO, 2012 THE NATIONAL ASSEMBLY
MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ANNEX 1C: AGREEMENT ON TRADE-RELATED ASPECTS OF INTELLECTUAL PROPERTY RIGHTS *
 International Investment Instruments: A Compendium MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ANNEX 1C: AGREEMENT ON TRADE-RELATED ASPECTS OF INTELLECTUAL PROPERTY RIGHTS * The Agreement
International Investment Instruments: A Compendium MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ANNEX 1C: AGREEMENT ON TRADE-RELATED ASPECTS OF INTELLECTUAL PROPERTY RIGHTS * The Agreement
LAW ON THE PROTECTION OF INVENTIONS. No. 50-XVI of March 7, Monitorul Oficial nr /455 din * * * TABLE OF CONTENTS.
 Translation from Romanian LAW ON THE PROTECTION OF INVENTIONS No. 50-XVI of March 7, 2008 Monitorul Oficial nr.117-119/455 din 04.07.2008 * * * TABLE OF CONTENTS Chapter I General Provisions Article 1.
Translation from Romanian LAW ON THE PROTECTION OF INVENTIONS No. 50-XVI of March 7, 2008 Monitorul Oficial nr.117-119/455 din 04.07.2008 * * * TABLE OF CONTENTS Chapter I General Provisions Article 1.
ANNEX XV REFERRED TO IN ARTICLE 7 PROTECTION OF INTELLECTUAL PROPERTY
 ANNEX XV REFERRED TO IN ARTICLE 7 PROTECTION OF INTELLECTUAL PROPERTY ANNEX XV REFERRED TO IN ARTICLE 7 PROTECTION OF INTELLECTUAL PROPERTY SECTION I GENERAL PROVISIONS Article 1 Definition of Intellectual
ANNEX XV REFERRED TO IN ARTICLE 7 PROTECTION OF INTELLECTUAL PROPERTY ANNEX XV REFERRED TO IN ARTICLE 7 PROTECTION OF INTELLECTUAL PROPERTY SECTION I GENERAL PROVISIONS Article 1 Definition of Intellectual
The Consolidate Patents Act
 The Consolidate Patents Act Publication of the Patents Act, cf. Consolidated Act No. 366 of 9 June 1998 as amended by Act No. 412 of 31 May 2000 TABLE OF CONTENTS Sections Part 1: General Provisions...
The Consolidate Patents Act Publication of the Patents Act, cf. Consolidated Act No. 366 of 9 June 1998 as amended by Act No. 412 of 31 May 2000 TABLE OF CONTENTS Sections Part 1: General Provisions...
Utility Models Act. Passed RT I 1994, 25, 407 Entry into force
 Issuer: Riigikogu Type: act In force from: 01.01.2015 In force until: In force Translation published: 23.12.2014 Amended by the following acts Passed 16.03.1994 RT I 1994, 25, 407 Entry into force 23.05.1994
Issuer: Riigikogu Type: act In force from: 01.01.2015 In force until: In force Translation published: 23.12.2014 Amended by the following acts Passed 16.03.1994 RT I 1994, 25, 407 Entry into force 23.05.1994
PRE-GRANT OPPOSITION POST-GRANT OPPOSITION
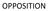 OPPOSITION TYPES OF OPPOSITION PRE-GRANT OPPOSITION [SEC 25(1)] POST-GRANT OPPOSITION [SEC. 25 (2)] REVOCATION[SECs 64 TO 66] GROUNDS FOR OPPOSITION UNDER SECTIONS 25(1) & 25 (2) That the applicant for
OPPOSITION TYPES OF OPPOSITION PRE-GRANT OPPOSITION [SEC 25(1)] POST-GRANT OPPOSITION [SEC. 25 (2)] REVOCATION[SECs 64 TO 66] GROUNDS FOR OPPOSITION UNDER SECTIONS 25(1) & 25 (2) That the applicant for
THE INDUSTRIAL PROPERTY BILL (No... of 2016) Explanatory Memorandum
 THE INDUSTRIAL PROPERTY BILL (No... of 2016) Explanatory Memorandum The main object of this Bill is to bring together in one enactment the provisions of the law relating to the protection of industrial
THE INDUSTRIAL PROPERTY BILL (No... of 2016) Explanatory Memorandum The main object of this Bill is to bring together in one enactment the provisions of the law relating to the protection of industrial
Patent Act, B.E (1979) As Amended until Patent Act (No.3), B.E (1999) Translation
 Patent Act, B.E. 2522 (1979) As Amended until Patent Act (No.3), B.E. 2542 (1999) Translation BHUMIBOL ADULYADEJ, REX. Given on the 11th day of March, B.E. 2522; Being the 34th year of the present Reign
Patent Act, B.E. 2522 (1979) As Amended until Patent Act (No.3), B.E. 2542 (1999) Translation BHUMIBOL ADULYADEJ, REX. Given on the 11th day of March, B.E. 2522; Being the 34th year of the present Reign
Law on the protection of inventions No. 50/2008 of the Republic of Moldova can be found at:
 The answers to this questionnaire have been provided on behalf of: Country: Republic of Moldova... Office: The State Agency on Intellectual Property... Person to be contacted: Name: Cicinova Olga... Title:
The answers to this questionnaire have been provided on behalf of: Country: Republic of Moldova... Office: The State Agency on Intellectual Property... Person to be contacted: Name: Cicinova Olga... Title:
New IP Code changes regarding patents, new post-grant opposition and enforcement provisions
 INTELLECTUAL PROPERTY - TURKEY New IP Code changes regarding patents, new post-grant opposition and enforcement provisions AUTHORS Mehmet Nazim Aydin Deriş January 08 2018 Contributed by Deris Avukatlik
INTELLECTUAL PROPERTY - TURKEY New IP Code changes regarding patents, new post-grant opposition and enforcement provisions AUTHORS Mehmet Nazim Aydin Deriş January 08 2018 Contributed by Deris Avukatlik
General Information Concerning. of IndusTRIal designs
 General Information Concerning Patents The ReGIsTRaTIon For Inventions of IndusTRIal designs 1 2 CONTENTS INTRODUCTION 3 1. What is a patent? 4 2. How long does a patent last? 4 3. Why patent inventions?
General Information Concerning Patents The ReGIsTRaTIon For Inventions of IndusTRIal designs 1 2 CONTENTS INTRODUCTION 3 1. What is a patent? 4 2. How long does a patent last? 4 3. Why patent inventions?
SWITZERLAND Patent Law as last amended on March 20, 2009 ENTRY INTO FORCE: January 1, 2012
 SWITZERLAND Patent Law as last amended on March 20, 2009 ENTRY INTO FORCE: January 1, 2012 TABLE OF CONTENTS First Title General Provisions Section 1 Requirements for Obtaining a Patent and Effects of
SWITZERLAND Patent Law as last amended on March 20, 2009 ENTRY INTO FORCE: January 1, 2012 TABLE OF CONTENTS First Title General Provisions Section 1 Requirements for Obtaining a Patent and Effects of
People s Republic of China State Intellectual Property Office of China
 [English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Office: People s Republic of China
[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Office: People s Republic of China
ANNEX VII REFERRED TO IN ARTICLE 25 PROTECTION OF INTELLECTUAL PROPERTY
 ANNEX VII REFERRED TO IN ARTICLE 25 PROTECTION OF INTELLECTUAL PROPERTY ANNEX VII REFERRED TO IN ARTICLE 25 PROTECTION OF INTELLECTUAL PROPERTY SECTION I GENERAL PROVISIONS Article 1 Definition of Intellectual
ANNEX VII REFERRED TO IN ARTICLE 25 PROTECTION OF INTELLECTUAL PROPERTY ANNEX VII REFERRED TO IN ARTICLE 25 PROTECTION OF INTELLECTUAL PROPERTY SECTION I GENERAL PROVISIONS Article 1 Definition of Intellectual
How to obtain PATENT and TRADEMARKS in India. JIII and AIPC. Brinda Mohan, India
 How to obtain PATENT and TRADEMARKS in India by JIII and AIPC Brinda Mohan, India Mohan Associates Advocates, Patents and Trademark Attorneys. D 4, III FLOOR CEEBROS BUILDING 11, CENETOPH ROAD TEYNAMPET
How to obtain PATENT and TRADEMARKS in India by JIII and AIPC Brinda Mohan, India Mohan Associates Advocates, Patents and Trademark Attorneys. D 4, III FLOOR CEEBROS BUILDING 11, CENETOPH ROAD TEYNAMPET
DRAFT PATENT LAW OF GEORGIA CHAPTER I. GENERAL PROVISIONS
 DRAFT PATENT LAW OF GEORGIA CHAPTER I. GENERAL PROVISIONS ARTICLE 1 This Law regulates property and personal non-property relations formed in connection with the creation, legal protection and usage of
DRAFT PATENT LAW OF GEORGIA CHAPTER I. GENERAL PROVISIONS ARTICLE 1 This Law regulates property and personal non-property relations formed in connection with the creation, legal protection and usage of
Intellectual Property Laws Amendment Act 2015
 Intellectual Property Laws Amendment Act 2015 No. 8, 2015 An Act to amend legislation relating to intellectual property, and for related purposes Note: An electronic version of this Act is available in
Intellectual Property Laws Amendment Act 2015 No. 8, 2015 An Act to amend legislation relating to intellectual property, and for related purposes Note: An electronic version of this Act is available in
ROMANIA Patent Law NO.64/1991 OFFICIAL GAZETTE OF ROMANIA, PART I, NO.613/19 AUGUST 2014
 ROMANIA Patent Law NO.64/1991 OFFICIAL GAZETTE OF ROMANIA, PART I, NO.613/19 AUGUST 2014 TABLE OF CONTENTS CHAPTER I - GENERAL PROVISIONS Art. 1 Art. 2 Art. 3 Art. 4 Art. 5 CHAPTER II - PATENTABLE INVENTIONS
ROMANIA Patent Law NO.64/1991 OFFICIAL GAZETTE OF ROMANIA, PART I, NO.613/19 AUGUST 2014 TABLE OF CONTENTS CHAPTER I - GENERAL PROVISIONS Art. 1 Art. 2 Art. 3 Art. 4 Art. 5 CHAPTER II - PATENTABLE INVENTIONS
AZERBAIJAN Law on Patent Date of Text (Enacted): July 25, 1997 ENTRY INTO FORCE: August 2, 1997
 AZERBAIJAN Law on Patent Date of Text (Enacted): July 25, 1997 ENTRY INTO FORCE: August 2, 1997 TABLE OF CONTENTS Chapter I General Provisions Article 1 Basic notions Article 2 Legislation of the Republic
AZERBAIJAN Law on Patent Date of Text (Enacted): July 25, 1997 ENTRY INTO FORCE: August 2, 1997 TABLE OF CONTENTS Chapter I General Provisions Article 1 Basic notions Article 2 Legislation of the Republic
PATENT LAW OF GEORGIA CHAPTER I. GENERAL PROVISIONS
 PATENT LAW OF GEORGIA CHAPTER I. GENERAL PROVISIONS ARTICLE 1 This Law regulates property and personal non-property relations formed in connection with the creation, legal protection and usage of the industrial
PATENT LAW OF GEORGIA CHAPTER I. GENERAL PROVISIONS ARTICLE 1 This Law regulates property and personal non-property relations formed in connection with the creation, legal protection and usage of the industrial
TREATY SERIES 2008 Nº 4. Act revising the Convention on the Grant of European Patents
 TREATY SERIES 2008 Nº 4 Act revising the Convention on the Grant of European Patents Done at Munich on 29 November 2000 Ireland s instrument of accession deposited with the Government of Germany on 16
TREATY SERIES 2008 Nº 4 Act revising the Convention on the Grant of European Patents Done at Munich on 29 November 2000 Ireland s instrument of accession deposited with the Government of Germany on 16
Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) (1994)*
 WORLD TRADE ORGANIZATION (WTO) Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) (1994)* TABLE OF CONTENTS** Article Part I: Part II: Section 1: Section 2: Section 3:
WORLD TRADE ORGANIZATION (WTO) Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) (1994)* TABLE OF CONTENTS** Article Part I: Part II: Section 1: Section 2: Section 3:
Intellectual Property Department Hong Kong, China. Contents
 Intellectual Property Department Hong Kong, China Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 3 Section 3: Experimental use and/or scientific research... 3 Section
Intellectual Property Department Hong Kong, China Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 3 Section 3: Experimental use and/or scientific research... 3 Section
[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights
![[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights [English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights](/thumbs/90/103895209.jpg) [English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Office: Dominican Republic... National
[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Office: Dominican Republic... National
Regulations to the Norwegian Patents Act (The Patent Regulations)
 Regulations to the Norwegian Patents Act (The Patent Regulations) This is an unofficial translation of the regulations to the Norwegian Patents Act. Should there be any differences between this translation
Regulations to the Norwegian Patents Act (The Patent Regulations) This is an unofficial translation of the regulations to the Norwegian Patents Act. Should there be any differences between this translation
A I P P I ASSOCIATION INTERNATIONALE POUR LA PROTECTION DE LA PROPRIETE INTELLECTUELLE
 A I P P I ASSOCIATION INTERNATIONALE POUR LA PROTECTION DE LA PROPRIETE INTELLECTUELLE INTERNATIONAL ASSOCIATION FOR THE PROTECTION OF INTELLECTUAL PROPERTY INTERNATIONALE VEREINIGUNG FÜR DEN SCHUTZ DES
A I P P I ASSOCIATION INTERNATIONALE POUR LA PROTECTION DE LA PROPRIETE INTELLECTUELLE INTERNATIONAL ASSOCIATION FOR THE PROTECTION OF INTELLECTUAL PROPERTY INTERNATIONALE VEREINIGUNG FÜR DEN SCHUTZ DES
PART I IMPLEMENTING REGULATIONS TO PART I OF THE CONVENTION
 EUROPEAN PATENT OFFICE Implementing Regulations to the Convention on the grant of European Patents as last amended on 15 October 2014 enter into force on 1 April 2015 TABLE OF CONTENTS PART I IMPLEMENTING
EUROPEAN PATENT OFFICE Implementing Regulations to the Convention on the grant of European Patents as last amended on 15 October 2014 enter into force on 1 April 2015 TABLE OF CONTENTS PART I IMPLEMENTING
OFFICIAL GAZETTE OF ROMANIA, PART I, NO.613/19 AUGUST 2014 REPUBLICATION PATENT LAW NO.64/1991 1
 OFFICIAL GAZETTE OF ROMANIA, PART I, NO.613/19 AUGUST 2014 REPUBLICATION PATENT LAW NO.64/1991 1 CHAPTER I - GENERAL PROVISIONS Art. 1 - (1) The rights in inventions shall be recognized and protected on
OFFICIAL GAZETTE OF ROMANIA, PART I, NO.613/19 AUGUST 2014 REPUBLICATION PATENT LAW NO.64/1991 1 CHAPTER I - GENERAL PROVISIONS Art. 1 - (1) The rights in inventions shall be recognized and protected on
REPUBLIC OF VANUATU BILL FOR THE PATENTS ACT NO. OF 1999
 REPUBLIC OF VANUATU BILL FOR THE PATENTS ACT NO. OF 1999 Arrangement of Sections PART 1 PRELIMINARY PROVISIONS 1. Interpretation PART 2 PATENTABILITY 2. Patentable invention 3. Inventions not patentable
REPUBLIC OF VANUATU BILL FOR THE PATENTS ACT NO. OF 1999 Arrangement of Sections PART 1 PRELIMINARY PROVISIONS 1. Interpretation PART 2 PATENTABILITY 2. Patentable invention 3. Inventions not patentable
Questionnaire on Exceptions and Limitations to Patent Rights. The answers to this questionnaire have been provided on behalf of:
 The answers to this questionnaire have been provided on behalf of: Country: Office: Republic of Poland Patent Office of the Republic of Poland Person to be contacted: Name: Piotr Czaplicki Title: Director,
The answers to this questionnaire have been provided on behalf of: Country: Office: Republic of Poland Patent Office of the Republic of Poland Person to be contacted: Name: Piotr Czaplicki Title: Director,
DRAFT REPORT. EN United in diversity EN 2012/2135(INI)
 EUROPEAN PARLIAMT 2009-2014 Committee on Development 25.7.2012 2012/2135(INI) DRAFT REPORT on development aspects of intellectual property rights on genetic resources: the impact on poverty reduction in
EUROPEAN PARLIAMT 2009-2014 Committee on Development 25.7.2012 2012/2135(INI) DRAFT REPORT on development aspects of intellectual property rights on genetic resources: the impact on poverty reduction in
Additional Features of New Patent Ordinance
 Additional Features of New Patent Ordinance Wednesday, January 19, 2005 08:00 IST Dr. Gopakumar G. Nair The Patent (Amendment) Ordinance 2004 and the Patent (Amendment) Rules 2005 notified on 26th December
Additional Features of New Patent Ordinance Wednesday, January 19, 2005 08:00 IST Dr. Gopakumar G. Nair The Patent (Amendment) Ordinance 2004 and the Patent (Amendment) Rules 2005 notified on 26th December
of 25 June 1954 (Status as of 1 January 2017) para. 2) is not patentable as an invention. 7
 English is not an official language of the Swiss Confederation. This translation is provided for information purposes only and has no legal force. Federal Act on Patents for Inventions (Patents Act, PatA)
English is not an official language of the Swiss Confederation. This translation is provided for information purposes only and has no legal force. Federal Act on Patents for Inventions (Patents Act, PatA)
NIGERIA Patents and Designs Act Chapter 344, December 1, 1971 Laws of the Federation of Nigeria 1990
 NIGERIA Patents and Designs Act Chapter 344, December 1, 1971 Laws of the Federation of Nigeria 1990 TABLE OF CONTENTS Patents 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Designs 12. 13. 14. 15. 16. 17. 18. 19.
NIGERIA Patents and Designs Act Chapter 344, December 1, 1971 Laws of the Federation of Nigeria 1990 TABLE OF CONTENTS Patents 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Designs 12. 13. 14. 15. 16. 17. 18. 19.
Order on Patents and Supplementary Protection Certificates
 1 The Patent and Trademark Office Order No. 25 of 18 January 2013 Order on Patents and Supplementary Protection Certificates Pursuant to section 5(2), section 6(2), section 8a, section 8b(2), section 9,
1 The Patent and Trademark Office Order No. 25 of 18 January 2013 Order on Patents and Supplementary Protection Certificates Pursuant to section 5(2), section 6(2), section 8a, section 8b(2), section 9,
Patent Law in Cambodia
 Patent Law in Cambodia September 2012 No 64, St 111 PO Box 172 Phnom Penh Cambodia +855 23 217 510 +855 23 212 740 +855 23 212 840 info@bnglegal.com www.bnglegal.com Patent Law in Cambodia September 2012
Patent Law in Cambodia September 2012 No 64, St 111 PO Box 172 Phnom Penh Cambodia +855 23 217 510 +855 23 212 740 +855 23 212 840 info@bnglegal.com www.bnglegal.com Patent Law in Cambodia September 2012
ANNEX V REFERRED TO IN ARTICLE 23 PROTECTION OF INTELLECTUAL PROPERTY
 ANNEX V REFERRED TO IN ARTICLE 23 PROTECTION OF INTELLECTUAL PROPERTY ANNEX V REFERRED TO IN ARTICLE 23 PROTECTION OF INTELLECTUAL PROPERTY Article 1 Intellectual property "Intellectual property" comprises
ANNEX V REFERRED TO IN ARTICLE 23 PROTECTION OF INTELLECTUAL PROPERTY ANNEX V REFERRED TO IN ARTICLE 23 PROTECTION OF INTELLECTUAL PROPERTY Article 1 Intellectual property "Intellectual property" comprises
MULTILATERAL TRADE NEGOTIATIONS THE URUGUAY ROUND
 MULTILATERAL TRADE NEGOTIATIONS THE URUGUAY ROUND RESTRICTED 7 July 1988 Special Distribution Group of Negotiations on Goods (GATI) Negotiating Group on Trade-Related Aspects of Intellectual Property Rights,
MULTILATERAL TRADE NEGOTIATIONS THE URUGUAY ROUND RESTRICTED 7 July 1988 Special Distribution Group of Negotiations on Goods (GATI) Negotiating Group on Trade-Related Aspects of Intellectual Property Rights,
WIPO Sub-Regional Workshop on Patent Policy and its Legislative Implementation
 WIPO Sub-Regional Workshop on Patent Policy and its Legislative Implementation Topic 12: Patent-related provisions in the framework of preferential trade agreements Marco M. ALEMAN Deputy Director, Patent
WIPO Sub-Regional Workshop on Patent Policy and its Legislative Implementation Topic 12: Patent-related provisions in the framework of preferential trade agreements Marco M. ALEMAN Deputy Director, Patent
DENMARK Patents Regulations Order No. 25 of 18 January, 2013 ENTRY INTO FORCE: 1 February, 2013
 DENMARK Patents Regulations Order No. 25 of 18 January, 2013 ENTRY INTO FORCE: 1 February, 2013 TABLE OF CONTENTS Part I Patent applications Chapter 1 Scope 1. Chapter 2 The contents and filing of applications
DENMARK Patents Regulations Order No. 25 of 18 January, 2013 ENTRY INTO FORCE: 1 February, 2013 TABLE OF CONTENTS Part I Patent applications Chapter 1 Scope 1. Chapter 2 The contents and filing of applications
HUNGARY Utility Model Act Act XXXVIII OF 1991 on the protection of utility models as consolidated on April 1, 2013
 HUNGARY Utility Model Act Act XXXVIII OF 1991 on the protection of utility models as consolidated on April 1, 2013 TABLE OF CONTENTS Chapter I SUBJECT MATTER OF AND RIGHTS CONFERRED BY UTILITY MODEL PROTECTION
HUNGARY Utility Model Act Act XXXVIII OF 1991 on the protection of utility models as consolidated on April 1, 2013 TABLE OF CONTENTS Chapter I SUBJECT MATTER OF AND RIGHTS CONFERRED BY UTILITY MODEL PROTECTION
Notification PART I CHAPTER I PRELIMINARY
 [TO BE PUBLISHED IN THE GAZZETTE OF INDIA, EXTRAORDINARY, PART II, SECTION 3, SUB-SECTION (i)] GOVERNMENT OF INDIA MINISTRY OF COMMERCE AND INDUSTRY (DEPARTMENT OF INDUSTRIAL POLICY AND PROMOTION) Notification
[TO BE PUBLISHED IN THE GAZZETTE OF INDIA, EXTRAORDINARY, PART II, SECTION 3, SUB-SECTION (i)] GOVERNMENT OF INDIA MINISTRY OF COMMERCE AND INDUSTRY (DEPARTMENT OF INDUSTRIAL POLICY AND PROMOTION) Notification
4. COMPARISON OF THE INDIAN PATENT LAW WITH THE PATENT LAWS IN U.S., EUROPE AND CHINA
 4. COMPARISON OF THE INDIAN PATENT LAW WITH THE PATENT LAWS IN U.S., EUROPE AND CHINA Provisions of the Indian patent law were compared with the relevant provisions of the patent laws in U.S., Europe and
4. COMPARISON OF THE INDIAN PATENT LAW WITH THE PATENT LAWS IN U.S., EUROPE AND CHINA Provisions of the Indian patent law were compared with the relevant provisions of the patent laws in U.S., Europe and
Exclusions from patentability 15 Inventions contrary to public order or morality not patentable
 New Zealand Patents Act 2013 Public Act 2013 No 68 Date of assent 13 September 2013 Reprint as at 14 September 2017 TABLE OF CONTENTS 1 Title 2 Commencement Part 1 Preliminary Purposes and overview 3 Purposes
New Zealand Patents Act 2013 Public Act 2013 No 68 Date of assent 13 September 2013 Reprint as at 14 September 2017 TABLE OF CONTENTS 1 Title 2 Commencement Part 1 Preliminary Purposes and overview 3 Purposes
Official Journal of the European Union L 251/3
 24.9.2009 Official Journal of the European Union L 251/3 COMMISSION REGULATION (EC) No 874/2009 of 17 September 2009 establishing implementing rules for the application of Council Regulation (EC) No 2100/94
24.9.2009 Official Journal of the European Union L 251/3 COMMISSION REGULATION (EC) No 874/2009 of 17 September 2009 establishing implementing rules for the application of Council Regulation (EC) No 2100/94
Questionnaire on Exceptions and Limitations to Patent Rights. The answers to this questionnaire have been provided on behalf of:
 Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Australia... Office: IP Australia... Person to be contacted: Name:
Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Australia... Office: IP Australia... Person to be contacted: Name:
TRADE MARKS ACT, 1999
 GOVERNMENT OF THE PEOPLE S REPUBLIC OF BANGLADESH A DRAFT BILL OF THE PROPOSED TRADE MARKS ACT, 1999 Prepared in the light of the complete report made by the Bangladesh Law Commission recommending promulgation
GOVERNMENT OF THE PEOPLE S REPUBLIC OF BANGLADESH A DRAFT BILL OF THE PROPOSED TRADE MARKS ACT, 1999 Prepared in the light of the complete report made by the Bangladesh Law Commission recommending promulgation
Revision Draft of the Patent Law of the People s Republic of China (For Deliberation)
 Revision Draft of the Patent Law of the People s Republic of China (For Deliberation) (Words in bold font are revised portion) Chapter 1: General Provisions Article 1 This law is enacted for the purpose
Revision Draft of the Patent Law of the People s Republic of China (For Deliberation) (Words in bold font are revised portion) Chapter 1: General Provisions Article 1 This law is enacted for the purpose
The Consolidate Utility Models Act 1)
 Consolidate Act No. 220 of 26 February 2017 The Consolidate Utility Models Act 1) Publication of the Utility Models Act, cf. Consolidate Act No. 190 of 1 March 2016 including the amendments which follow
Consolidate Act No. 220 of 26 February 2017 The Consolidate Utility Models Act 1) Publication of the Utility Models Act, cf. Consolidate Act No. 190 of 1 March 2016 including the amendments which follow
Implementing Regulations to the Convention on the Grant of European Patents
 Implementing Regulations to the Convention on the Grant of European Patents of 5 October 1973 as adopted by decision of the Administrative Council of the European Patent Organisation of 7 December 2006
Implementing Regulations to the Convention on the Grant of European Patents of 5 October 1973 as adopted by decision of the Administrative Council of the European Patent Organisation of 7 December 2006
ANNEX VI REFERRED TO IN ARTICLE 24 PROTECTION OF INTELLECTUAL PROPERTY
 ANNEX VI REFERRED TO IN ARTICLE 24 PROTECTION OF INTELLECTUAL PROPERTY ANNEX VI REFERRED TO IN ARTICLE 24 PROTECTION OF INTELLECTUAL PROPERTY TITLE I GENERAL PROVISIONS Article 1 Definition of Intellectual
ANNEX VI REFERRED TO IN ARTICLE 24 PROTECTION OF INTELLECTUAL PROPERTY ANNEX VI REFERRED TO IN ARTICLE 24 PROTECTION OF INTELLECTUAL PROPERTY TITLE I GENERAL PROVISIONS Article 1 Definition of Intellectual
From Law of Patents, Layout Designs of Integrated Circuits, Plant Varieties, and Industrial Designs, Chapter Two:
 Saudi Patent Office Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 2 Section 3: Experimental use and/or scientific research... 3 Section 4: Preparation of medicines...
Saudi Patent Office Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 2 Section 3: Experimental use and/or scientific research... 3 Section 4: Preparation of medicines...
[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights
![[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights [English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights](/thumbs/86/94777095.jpg) [English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Costa Rica... Office: Industrial Property
[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: Costa Rica... Office: Industrial Property
Questionnaire on Exceptions and Limitations to Patent Rights. The answers to this questionnaire have been provided on behalf of:
 The answers to this questionnaire have been provided on behalf of: Country: Austria... Office: Austrian Patent Office (APO)... Person to be contacted: Name:... Title:... E-mail:... Telephone:... Facsimile:...
The answers to this questionnaire have been provided on behalf of: Country: Austria... Office: Austrian Patent Office (APO)... Person to be contacted: Name:... Title:... E-mail:... Telephone:... Facsimile:...
RUSSIA Patent Law #3517-I of September 23, 1992, as amended by the federal law 22-FZ of February 7, 2003 ENTRY INTO FORCE: March 11, 2003
 RUSSIA Patent Law #3517-I of September 23, 1992, as amended by the federal law 22-FZ of February 7, 2003 ENTRY INTO FORCE: March 11, 2003 TABLE OF CONTENTS Section I General Provisions Article 1 Relations
RUSSIA Patent Law #3517-I of September 23, 1992, as amended by the federal law 22-FZ of February 7, 2003 ENTRY INTO FORCE: March 11, 2003 TABLE OF CONTENTS Section I General Provisions Article 1 Relations
Utility Model Law I. GENERAL PROVISIONS
 Utility Model Law Federal Law Gazette 1994/211 as amended by Federal Law Gazette I 1998/175, I 2001/143, I 2004/149, I 2005/42, I 2005/130, I 2005/151, I 2007/81 and I 2009/126 I. GENERAL PROVISIONS Subject
Utility Model Law Federal Law Gazette 1994/211 as amended by Federal Law Gazette I 1998/175, I 2001/143, I 2004/149, I 2005/42, I 2005/130, I 2005/151, I 2007/81 and I 2009/126 I. GENERAL PROVISIONS Subject
of Laws for Electronic Access SLOVAKIA Law on Inventions, Industrial Designs and Rationalization Proposals (No. 527 of November 27, 1990)*
 Law on Inventions, Industrial Designs and Rationalization Proposals (No. 527 of November 27, 1990)* TABLE OF CONTENTS** Sections Purpose of the Law... 1 Part One: Inventions Chapter I: Patents... 2 Patentability
Law on Inventions, Industrial Designs and Rationalization Proposals (No. 527 of November 27, 1990)* TABLE OF CONTENTS** Sections Purpose of the Law... 1 Part One: Inventions Chapter I: Patents... 2 Patentability
THE PROTECTION OF NEW VARIETIES OF PLANTS ACT Official consolidated text (ZVNSR-UPB1)
 On the basis of Article 153 of the Rules of Procedure of the National Assembly, the National Assembly of the Republic of Slovenia has at its session of 29 September 2005 approved official consolidated
On the basis of Article 153 of the Rules of Procedure of the National Assembly, the National Assembly of the Republic of Slovenia has at its session of 29 September 2005 approved official consolidated
Protection of Plant Varieties in Egypt: Law
 Protection of Plant Varieties in Egypt: Law 82-2002 Nadia Kholeif I. Introduction Many countries have not traditionally provided patent protection for living matter plant varieties, microorganisms, and
Protection of Plant Varieties in Egypt: Law 82-2002 Nadia Kholeif I. Introduction Many countries have not traditionally provided patent protection for living matter plant varieties, microorganisms, and
Regional Comprehensive Economic Partnership. Intellectual Property Chapter and the Impact on Access to Medicines
 MSF RCEP IP Chapter Technical Analysis NOVEMBER 2016 Regional Comprehensive Economic Partnership Intellectual Property Chapter and the Impact on Access to Medicines This briefing note details Médecins
MSF RCEP IP Chapter Technical Analysis NOVEMBER 2016 Regional Comprehensive Economic Partnership Intellectual Property Chapter and the Impact on Access to Medicines This briefing note details Médecins
FINLAND Patents Decree No. 669 of September 26, 1980 as last amended by Decree No. 580 of 18 July 2013 Enter into force on 1 September 2013
 FINLAND Patents Decree No. 669 of September 26, 1980 as last amended by Decree No. 580 of 18 July 2013 Enter into force on 1 September 2013 TABLE OF CONTENTS Patent Application and Record of Applications
FINLAND Patents Decree No. 669 of September 26, 1980 as last amended by Decree No. 580 of 18 July 2013 Enter into force on 1 September 2013 TABLE OF CONTENTS Patent Application and Record of Applications
Central Government Act The Trade And Merchandise Marks Act, 1958
 Central Government Act The Trade And Merchandise Marks Act, 1958 THE TRADE AND MERCHANDISE MARKS ACT, 1958 ACT NO. 43 OF 1958 [ 17th October, 1958.] An Act to provide for the registration and better protection
Central Government Act The Trade And Merchandise Marks Act, 1958 THE TRADE AND MERCHANDISE MARKS ACT, 1958 ACT NO. 43 OF 1958 [ 17th October, 1958.] An Act to provide for the registration and better protection
CHAPTER 2 AUTHORS AND PATENT OWNERS Article 5. Author of the Invention, Utility Model, and Industrial Design Article 6.
 BELARUS Law of the Republic of Belarus On Patents for Inventions, Utility Models, and Industrial Designs December 16, 2002 No 160-Z Amended as of December 22, 2011 TABLE OF CONTENTS CHAPTER 1. LEGAL PROTECTION
BELARUS Law of the Republic of Belarus On Patents for Inventions, Utility Models, and Industrial Designs December 16, 2002 No 160-Z Amended as of December 22, 2011 TABLE OF CONTENTS CHAPTER 1. LEGAL PROTECTION
Dahir No of 9 Kaada 1420 (February 15, 2000) on the Enactment of Law No on the Protection of Industrial Property
 Dahir No. 1-00-91 of 9 Kaada 1420 (February 15, 2000) on the Enactment of Law No. 17-97 on the Protection of Industrial Property TABLE OF CONTENTS Articles Title I: Title II: Chapter I: Chapter II: Section
Dahir No. 1-00-91 of 9 Kaada 1420 (February 15, 2000) on the Enactment of Law No. 17-97 on the Protection of Industrial Property TABLE OF CONTENTS Articles Title I: Title II: Chapter I: Chapter II: Section
Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law
 !!! Dangers for Access to Medicines in the Trans-Pacific Partnership Agreement: Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law ! Issue US TPPA Proposal Andean Community
!!! Dangers for Access to Medicines in the Trans-Pacific Partnership Agreement: Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law ! Issue US TPPA Proposal Andean Community
[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights
![[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights [English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights](/thumbs/90/103707752.jpg) [English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: HONDURAS... Office: DIRECTORATE GENERAL
[English translation by WIPO] Questionnaire on Exceptions and Limitations to Patent Rights The answers to this questionnaire have been provided on behalf of: Country: HONDURAS... Office: DIRECTORATE GENERAL
Summary and Conclusions
 Summary and Conclusions In this thesis, results are presented of a study on the alignment of the European Patent Convention and the Patent Cooperation Treaty with requirements of the Patent Law Treaty.
Summary and Conclusions In this thesis, results are presented of a study on the alignment of the European Patent Convention and the Patent Cooperation Treaty with requirements of the Patent Law Treaty.
AUSTRIA Utility Model Law
 AUSTRIA Utility Model Law BGBl. No. 211/1994 as amended by BGBl. Nos. 175/1998, 143/2001, I 2004/149, I 2005/42, I 2005/130, I 2005/151, I 2007/81 and I 2009/126 TABLE OF CONTENTS I. GENERAL PROVISIONS
AUSTRIA Utility Model Law BGBl. No. 211/1994 as amended by BGBl. Nos. 175/1998, 143/2001, I 2004/149, I 2005/42, I 2005/130, I 2005/151, I 2007/81 and I 2009/126 TABLE OF CONTENTS I. GENERAL PROVISIONS
Act No. 435/2001 Coll. on Patents, Supplementary Protection Certificates and on Amendment of Some Acts as Amended (The Patent Act)
 Act No. 435/2001 Coll. on Patents, Supplementary Protection Certificates and on Amendment of Some Acts as Amended (The Patent Act) Amended by : Act No. 402/2002 Coll. Act No. 84/2007 Coll. Act No. 517/2007
Act No. 435/2001 Coll. on Patents, Supplementary Protection Certificates and on Amendment of Some Acts as Amended (The Patent Act) Amended by : Act No. 402/2002 Coll. Act No. 84/2007 Coll. Act No. 517/2007
Article 2: A patent of invention shall not be granted in respect of the following:
 Part One: Patents Chapter One: General Provisions Chapter Two: Procedure of Application for a Patent Chapter Three: Transfer of Ownership, Pledge, and Attachment of Patent Chapter Four: Compulsory Licensing
Part One: Patents Chapter One: General Provisions Chapter Two: Procedure of Application for a Patent Chapter Three: Transfer of Ownership, Pledge, and Attachment of Patent Chapter Four: Compulsory Licensing
