Effect of TRIPS on Pricing, Affordability and Access to Essential Medicines in Bhutan
|
|
|
- Kimberly Tyler
- 5 years ago
- Views:
Transcription
1 Effect of TRIPS on Pricing, Affordability and Access to Essential Medicines in Bhutan Abstract DR. TANDI DORJI ** Health care in Bhutan is free and the Essential Drugs Program under the Ministry of Health has been able to provide high quality effective medicines for the people. The bulk of these medicines is imported from generic companies in India, where based on its 1970 patent laws, copies of patented drugs were manufactured using different processes which made it cheap and affordable for many developing countries. However with the enactment in India of its new patent laws in 2005 and with Bhutan becoming a member of the WTO, the affordability of those medicines developed post-1995 will become severely limited. With both India and Bhutan becoming TRIPS compliant, we will have to incorporate and amend our national laws and review how best we can utilize the flexibilities in TRIPS, afforded by the Doha Declaration and the Decision of the General Council. We need to address these issues if we are to safeguard public health and to continue to access affordable high quality medicines for our people. Introduction With the small population of approximately 700,000, Bhutan has made significant strides in health since 1961, when the health department and the first hospital were established. 1 Health care is free for all citizens, delivered through public hospitals and dispensaries. Access to health care is good with 90 % of the population having access to a health center within 3 hours walking distance. 2 The Drugs, Vaccines and Equipments division (DVED) is responsible for the purchase and supply of all medicine, throughout the country. Printed with permission from the Centre for Bhutan Studies ** Pediatrician, JDWNR Hospital, Thimphu. 1 Planning Commission (1999). Bhutan A vision for peace, prosperity and happiness., Thimphu: Royal Government of Bhutan. 2 Department of Health Services (2000). A Report: National Health Survey 2000, Thimphu: Royal government of Bhutan.
2 2 Arellano Law and Policy Review Vol. 9 No. 1 In the 9th five year plan ( ) the ministry of health received 6.4 % of the total budget outlay, which is one of the highest in the region. 3 Like most developing countries procurement of pharmaceuticals accounted for the second highest expenditure. 4 The Essential Drug Program (EDP), which began in 1987, distributes medicines to different levels of care and is considered exemplary by the World Health Organization (WHO). 5 It noted that 90 % of people had access to high quality essential drugs, and only 0.75 % of the overall budget was wasted on expiry drugs. The National Drug Policy guides the procurement and supply of medicines usually from pre-qualified suppliers and through central procurement. The government was thus able to negotiate and purchase these essential medicines at a price that was 50% below the world market prices. 6 Regular prescription and drug utilization surveys enable pharmacists to interact closely with doctors and ensure that the medicines are put to their best use with minimum waste. The people thus benefit from these policies, with access to health care made available by the government and the EDP ensuring timely availability of high quality drugs. However with Bhutan on the verge of becoming a member of the World Trade Organization (WTO), we will have to comply with the TRIPS (Trade related aspects of intellectual property rights) agreement and this poses several challenges to accessing and being able to afford essential medicines. To aggravate the situation, India who is our biggest source of essential drugs, enacted its new patent laws and like other least developed countries (LDC), Bhutan will find it difficult to maintain the present system of providing free, cheap, high quality medicines. There have been several discussions from various sectors on the advantages and disadvantages of becoming a member of the WTO here in Bhutan, however very little has been discussed or written from the health perspective. This paper will therefore discuss the issue of access and 3 National Statistical Bureau (2004). Statistical Yearbook of Bhutan, 2003, Thimphu: Royal Government of Bhutan. 4 Hogerzeil, H.V. (2005). The concept of essential medicines: lessons for rich countries, British Medical Journal, Organization WH. Bhutan: Health infrastructure. In; Available at 6 Group TFW (2004). Millennium project: Access to Essential medicines, Geneva: United Nations Development Fund (UNDP).
3 November 2008 Effects of TRIPS in Pricing, Affordability to Essential Medicine 3 affordability of medicines in relationship to TRIPS, which is the major consequence for health on Bhutan becoming a member of the WTO. Background to TRIPS and its challenges to developing countries In 1994, when the WTO was formed, its member states adopted TRIPS as a means for securing intellectual property protection for pharmaceuticals and other technologies. The reason for this was to encourage funding and to provide incentives for big pharmaceutical companies to continue to research and develop new drugs. It costs between US $ million, 7 and takes years of research to put new medicines into the market. TRIPS enabled these major R&D pharmaceutical companies to recover their costs by way of patents. As per article 27 of the TRIPS agreement, patents on these new drugs provided exclusive rights to the producer and prevented others from making, using, offering for sale, selling or importing the new product for a period of 20 years, during which time they not only regained their cost but also made huge profits. Developing countries were given until 2000 to comply with TRIPS provisions and LDC s were given six additional years until 2006, which was subsequently extended to 2016 with respect to medicines. Although theoretically this seems fair, in reality this puts developing countries at a huge disadvantage. Drug companies are driven by profits and therefore tend to invest in developing medicines for diseases that are more prevalent in Western countries, mostly addressing the so called lifestyle diseases as these richer and bigger markets helped generate better profits. Diseases that are rampant in developing countries are neglected and it is for this reason that we have not seen any new drugs for diseases like tuberculosis, leishmaniasis, shigellosis and meningitis for the last three or four decades. Between there were 1,223 new chemical entities commercialized, out of which 379 (30.9 %) were considered as therapeutic innovations and only 13 were specific for tropical diseases and sadly only 4 were produced as a direct result of R & D conducted by pharmaceutical 7 DiMasi, J.A., Hansen, R.W. and Grabowski, H.G. (2003). The price of innovation: new estimates of drug development costs, J Health Econ, 22:
4 4 Arellano Law and Policy Review Vol. 9 No. 1 industries. 8 Even when new drugs are developed (e.g. artemesinin for malaria), they are too expensive to be affordable for a vast majority of people that need them. Developing countries on the other hand lack technical, financial and human resources to carry out research and develop new drugs, especially given that they must follow Good manufacturing practices. This is a process by which new drugs have to pass through various stages to ensure that it is effective, safe and of high quality before the drug is marketed. All these requirements lead to high production costs, thereby making patented drugs expensive and out of reach for the poorer section of the world. Up until 2005, India had followed its own patent laws established in 1970, which granted patents to processes only and not for products. Therefore by using different processes such as reverse engineering, India was able to produce generic versions of patented medicines and this lead to the growth of a huge generic industry, which supplies 70% of the world s generic medicines. In addition, for registration of these generics and to prove that the drug was bio-equivalent (of the same standard in terms of efficacy and quality) to the original patented drug, it did not have to pass through the same stringent process of testing and providing supportive data. It could use the evidence of the data submitted by the original company or proof of the original drugs registration by a stringent regulatory authority in another country. It also had the freedom to combine multiple patented drugs into a single tablet/capsule (there are no patents on fixed dose combinations) thereby making it convenient for the patients in terms of compliance. Since these drastically reduced the costs of production, generic medicines were much cheaper, leading to a major reduction in drug prices. The effects of these price differences were significantly seen with anti-retroviral medicines, which are used for treating HIV/AIDS patients. With the pandemic reversing the development achievements in many African nations and threatening to do the same in Asia, a reduction in prices of anti-hiv/aids drugs from an unaffordable US $ 10,000 (for treating one patient for a year) to an affordable US $ 140 was a boon and a blessing for many developing countries combating this disease. 8 Pecoul, B, Chirac, B, Trouller, P et al. Access to essential drugs in poor countries: a lost battle? JAMA. Available at:
5 November 2008 Effects of TRIPS in Pricing, Affordability to Essential Medicine 5 While the countries of the south, non-governmental organizations (NGO s), civil society and international humanitarian organizations welcomed this, the major drug companies were fuming, seeing their potential profits dwindle. They continued to exercise their influence with the support of their governments and pushed the WTO for more stringent measures to follow TRIPS protocol and on several occasions brought governments to the WTO for arbitration, which often ended in embarrassment for these companies. 9 At the same time, developing countries continued to fight for more recognition of public health concerns and better access to cheap, effective and high quality generic drugs. This conflict came to the forefront in November 2001 at Doha, Qatar, which was regarded by many as a victory for developing countries. The Doha Declaration of 2001 ensured access to cheap high quality generic drugs for diseases such as HIV, malaria and tuberculosis. It allowed countries such as India, Brazil and Thailand to use certain flexibilities within TRIPS, especially compulsory licenses, to continue to manufacture these essential drugs in the generic form. 10 Article 31 (f) of TRIPS however, stated that compulsory licenses for the manufacture of medicines were to be issued predominantly for the supply of the domestic market of the member. 11 Non-Producing Countries (NPC), like Bhutan, with no domestic pharmaceutical industry or market, cannot make use of compulsory licenses, and importing cheap generic drugs from India is the only lifeline. The only relevant comment for such countries from the declaration, is the instruction given to the Council for TRIPS to find an expeditious solution to this problem by Subsequently after nearly 2 years of debate in 2003, the General Council by way of its 30th August decision 12 agreed to waive article 31 (f) of the TRIPS agreement for 9 Brazil, Abbot, & AIDS drugs patents. Available at: 10 World Trade Organization (2001). Declaration on TRIPS agreement and public health. Ministerial declaration. Ministerial Conference, fourth session, 9-14 November 2001, DOHA. WT/MIN (01)/DEC/2, Available at: _e/minist_e/min01_e/mindecl_trips_e.pdf. 11 World Trade Organization. Agreement on Trade related aspects of intellectual property rights. Part II, Standards concerning the availability, scope and use of Intellectual property rights, Article 31 (f).available at: _e/trips_e/t_agm3c_e.htm. 12 WT/L/540 and Corr.1. Decision of the General Council of 30 August Implementation of paragraph 6 of the Doha Declaration on the TRIPS Agreement and
6 6 Arellano Law and Policy Review Vol. 9 No. 1 LDC s and this was further supplemented with a statement by the General council chairman in Although several concessions were made and many of the points clarified, it made implementation of the flexibilities cumbersome and impractical. 13 Countries intending to import or export medicines had to pursue several labyrinthine procedures and fulfill all the criteria as set out by the council making it unlikely for any member to use it effectively. Even if Bhutan attempts to meet the requirements mentioned in the decision, it is unlikely that the Indian companies willing to export drugs will take the initiative or the effort to fulfill their part of the criteria because of the myriad of labyrinthine procedures and the relatively small demand. 14 India s decision to become TRIPS compliant with the passing of the Indian patent act by parliament in March 2005 will further aggravate the situation. Amidst a walkout by the opposition party, the controversial law was passed, and what was disheartening for most developing countries was that the law went beyond what was required by TRIPS. 15 Under the new law, besides new chemicals and products, patents can also be given for formulations, new drug delivery systems and combinations, making it possible for companies to acquire patents on new uses of old drugs and on new combination of old drugs. This will severely restrict access to pharmaceuticals, even for those drugs that were made prior to 1995, which are exempt from patent laws. The new laws also make the exporting of compulsory licensed drugs illegal if the importing country does not have a license too. For Bhutan, which does not have to comply with TRIPS until 2016, and where there are no patents for any drugs, it seems absurd to issue a compulsory license for a non-existent patent. With limited financial resources and heavily dependent on donors, Bhutan will not be able to afford the more expensive patented drugs. This can have serious impact on the sustainability of the present provision of free medicines. public health. Available at: _para6_e.htm 13 Correa, C.M. (2004). Implementation of the WTO General Council decision on paragraph 6 of the DOHA declaration on TRIPS agreement and public health. Department of Essential drugs and medicine policy, Geneva: World Health Organization. 14 For example the number of HIV infected people is only 72 and there are a few hundred with tuberculosis and malaria. (Annual health bulletin Ministry of health, Royal government of Bhutan) 15 Sharma, D.C. (2005). Indian patents may hamper access to anti retroviral globally, The Lancet, 5: 136
7 November 2008 Effects of TRIPS in Pricing, Affordability to Essential Medicine 7 Effect of TRIPS on pricing, affordability and access Given its geographical location and rapid globalization, Bhutan reluctantly began its accession to the WTO in 2004, aware of the many pitfalls and the heavy disadvantages that it faced. 16 However, very little discussion took place with regards to TRIPS and public health, and especially with affordability and accessibility of drugs. The application of national laws can still assist poor countries in accessing affordable drugs, however with limited trained professionals in all sectors we are yet to address such issues and to amend and refine our trade laws, in particular those related to TRIPS. Even after establishing relevant laws, it will be essential to have a system in place and a common understanding between all sectors in safeguarding our rights to use the flexibilities because companies can still find ways to block the export of drugs, as was seen in Philippines, where after issuing compulsory license for 51 drugs, only 1 managed to be distributed and that too after 10 years of effort. 17 Bhutan also depends upon bilateral agencies and the UN for much of our revenue. For example, in 2000, 27.5% of our expenditure for public health came from external sources. 18 Even though we spend a high percentage of our budget on health, it will be difficult to sustain the delivery of free medicines and continue our policy of free health care. Insurance companies are unlikely to take over the financing of health care because the numbers of clients are likely to be small and because of the absence of private hospitals and medical facilities in Bhutan. Moreover, more and more patients, those who can afford to pay the premiums will seek care in third countries making such ventures for insurance companies non-viable. Therefore, unless alternatives are explored and tested, Bhutan will not be able to afford the patented drugs with the present budget. 16 Wangyel, Tashi (2004). Rhetoric and reality: An assessment of the possible impact of WTO on Bhutan, in The Spider and the Piglet, Thimphu: The Center of Bhutan studies. 17 Correa, C.M. (2004). Implementation of the WTO General Council decision on paragraph 6 of the DOHA declaration on TRIPS agreement and public health. Department of Essential drugs and medicine policy, Geneva: World Health Organization. 18 World Health Organization. Health resources: Bhutan. Available at org/cntryhealth/bhutan/bhuresource.htm.
8 8 Arellano Law and Policy Review Vol. 9 No. 1 Although health services will be accessible, the provision of free drugs may not be possible unless some mechanism of charging fees is developed. This will then lead to essential drugs becoming inaccessible to a majority of the population. Because poor people tend to pay out of pocket for medicines, there is a danger of having a 2-tier system of access, one having access to effective expensive drugs and the other to older, less effective, patent expired generics. 19 Options for utilizing the TRIPS flexibilities We are not required to be TRIPS compliant till 2016; however, since we do not have a domestic pharmaceutical industry, we are dependent on other countries, predominantly India, which has not only become TRIPS compliant but has also implemented its national laws incorporating these changes. It is for this reason that we need to address TRIPS and other issues in relation to public health so that we continue to safeguard the health of our people and ensure access to affordable and effective medicines. As a LDC/NPC, Bhutan is eligible to utilize the Para 6 Decision as an importer and to purchase generic medicines from any manufacturer. The only requirement as per the General Council August decision is for us to make a notification to the WTO in this regard. However for the exporter there are several procedures; seek voluntary license from the patent holder on commercially reasonable terms for a reasonable period, seek and obtain a compulsory license from its government, manufacture and export only the specified amount, pay royalties to the patent holder based on the commercial value in the importing country, investigate the patent holders product in the importing country and differentiate it significantly and prominently, seek registration and prove bio-equivalence to the regulatory authority in the importing country, and notify to the WTO along with postings of the entire detail on its website. This same procedure has to be replicated for every individual drug and for every country to which the exporter intends to export! Given these procedures, the consequent delay in production, and the cost implication, no generic manufacturer would be inclined to take the initiative, especially when you consider the small demand of the Bhutanese market. 19 Mudur, G. (2005). Changes to India s patent law may deny cheap drugs to millions, British Medical journal, 330: 692.
9 November 2008 Effects of TRIPS in Pricing, Affordability to Essential Medicine 9 For Bhutan, the options available from the TRIPS flexibilities are to import no-patent drugs (older) without restriction or to negotiate with exporting countries for issuing of compulsory license for export. There are no restrictions on importing medicines from a no-patent country, but where there are patents, such as in India, we can import either only nonpredominant amounts or an unlimited amount depending upon whether a ordinary compulsory license (Article 31 (f)) or a compulsory license to effect Article 31 (k) (related to patent abuse) is issued in the exporting country. Both of these are unlikely because of the limited commercial benefits for the drug manufacturer, an incapacity to reach economies of scale, and its adverse impact on foreign direct investment. Another option to consider is to seek and negotiate with the exporting country for an Article 30 Limited exception export, whereby it permits a pharmaceutical company to manufacture products for export to a no-patent country or in response to a compulsory license from a NPC. This is considered by many, including international organizations like the World Health Organization, to be the most efficient and expeditious way to access cheaper generic medicines. However, there is very limited experience in using this clause and it therefore may be considered risky by the manufacturer. It will also require strong legislative authority and the support of the government. Developed countries and the patent holder can still challenge this limited exception rule and bring the case before the WTO. But given the commercial risk, many exporters will not have enthusiasm for this route. Therefore, even though the Doha Declaration and the Decision of the Council promises to ease access to essential medicines for developing countries like Bhutan, in practical terms it will be more difficult to do so. The inability to utilize all the flexibilities afforded by these decisions, absence of strong national laws and legislations, and the fear of repercussion from powerful Western governments and lobbies pose serious threats to LDC s efforts to safeguard public health. Moreover, there is a constant danger of powerful countries adding TRIPS plus provisions into any bilateral or regional trade negotiations, which effectively deny these very flexibilities.
10 10 Arellano Law and Policy Review Vol. 9 No. 1 Recommendations The first and foremost action that we need to take in respect to TRIPS and public health is to develop national laws that incorporate TRIPS so as to safeguard the country s ability to import and deliver high quality drugs to its people. Regulations and legislations should address such issues as: granting of compulsory license to import drugs for government non-commercial use without prior notification; import and reexport within the region; registration of generic drugs and proof of bioequivalence; limiting patent holders rights of appeal; setting royalty rates; defining international exhaustion regimes; and above all to legally enable the EDP and the Ministry of Trade to resort to all the flexibilities in the TRIPS agreement and related texts. At the same time, laws to prevent the re-export of licensed generics should also be made and enforced in order to gain the confidence of patent holders. Consultation should be held between the Ministry of Health, the Ministry of Trade, the Foreign Ministry, the Royal Court of Justice, and the relevant international organizations that provide technical assistance. The waiver offered by the 2003 council meeting must be incorporated as a solution through urgently needed national laws. Secondly, negotiations with the Indian government through regional organizations, such as SAFTA (South Asian Free Trade Agreement), should be initiated soon in order to address ways to increase access to generic drugs. A regional approach would benefit both the manufacturer in terms of reaching economies of scale and the member countries as exemplified by the African Intellectual Property Organization. The decision of the General Council in paragraph 6 also encourages such co-operation and specifically mentions that Article 31(f) of the TRIPS Agreement shall be waived to the extent necessary to enable a pharmaceutical product produced or imported under a compulsory license in that Member to be exported to the markets of those other developing or least developed country parties to the regional trade agreement that share the health problem in question. Another mechanism that could be employed is to have a centralized pooled system of purchasing essential drugs. Pooled procurement, like the Eastern Caribbean Drug Service 20 helps member countries to purchase drugs from a single manufacturer and 20 Rouselle, M.F. and Burnett, F. Cost containment through pharmaceutical procurement: A Caribbean case study, International Journal of Health Planning and Management, 11 (2):
11 November 2008 Effects of TRIPS in Pricing, Affordability to Essential Medicine 11 because the bulk ordered is large, helps to negotiate prices and bring them down to affordable rates. LDC s do not have to comply with TRIPS until 2016, and so Bhutan should take full advantage of the flexibilities accorded and in the meantime develop a pool of professionals from the different departments to address all the issues of TRIPS. For the Ministry of Health and especially the procurement division of the EDP, it is important that key personnel are trained to address these issues nationally and internationally so that uncertainties about patent status do not create a barrier. They should also ensure that appropriate policies on selection, purchase, appropriate taxes and prescribing practices are drawn up so that these factors do not feature in the rise of drug prices. 21 Bhutan should continue to push for simpler and faster procedures to benefit from compulsory licenses, differential pricing and the waiver of article 31 (f). With other partners Bhutan should urge the WTO to explore new ways to benefit poorer countries, such as equity pricing, 22 automatic licensing and fixed royalties for patented drugs. 23 It should negotiate with India on parallel import mechanisms and also on immediate export with a notification from Bhutan, without the cumbersome procedure of getting a compulsory license in India Henry, D and Lexchin, J (2002). The pharmaceutical industry as a medicines provider, The Lancet, 360: Chaudhuri, S., Goldberg, P.K., and Jia, P. (2003). The effect of extending intellectual property rights protection to developing countries: A case study of the Indian pharmaceutical marke, National Bureau of Economic Research, working paper 10159, Cambridge. 23 Chaterjee, P. (2005). India s new patent may still hurt generic drug supplies, The lancet, 365: Ahmed, K. (2005). India s new patent bill threatens generic industry, The lancet, 5: 265.
12 12 Summary Arellano Law and Policy Review Vol. 9 No. 1 Bhutan has an effective EDP with more than 90% of its people having access to high quality medicines, mainly generics imported from India. With India s new patent laws, the sustainability of the EDP and free health care policy is threatened. Without a domestic pharmaceutical industry, a compulsory license is unlikely to benefit Bhutan and we should rely instead on the waiver issued by the council in It is important that Bhutan enact its own national laws to safeguard affordability and access to quality drugs and explore all the flexibilities accorded in the Doha Declaration. It should also build a strong technical professional team to address these issues nationally and internationally. It is important to forge ties with its neighbours within the region to set up mechanisms to protect the fundamental right of its citizens to quality health care. It must ensure that its citizens have access to quality and effective drugs at a cost which the country can afford. In this highly globalised and unequal world, this will only be possible through its own political will.
CRS Report for Congress
 Order Code RS21609 Updated November 5, 2003 CRS Report for Congress Received through the CRS Web The WTO, Intellectual Property Rights, and the Access to Medicines Controversy Summary Ian F. Fergusson
Order Code RS21609 Updated November 5, 2003 CRS Report for Congress Received through the CRS Web The WTO, Intellectual Property Rights, and the Access to Medicines Controversy Summary Ian F. Fergusson
WIPO-ESCAP-IIUM Regional Workshop on Intellectual Property and Public Health and Environment Policy for Asia and Pacific
 Intellectual Property and Public Health Cambodian Perspective WTO-ESCAP-IIUM REGIONAL WORKSHOP ON IP AND PUBLIC HEALTH AND ENVIRONMENT POLICY FOR ASIA AND PACIFIC REGION Kaula Lumpur, Malaysia 10-12 JULY
Intellectual Property and Public Health Cambodian Perspective WTO-ESCAP-IIUM REGIONAL WORKSHOP ON IP AND PUBLIC HEALTH AND ENVIRONMENT POLICY FOR ASIA AND PACIFIC REGION Kaula Lumpur, Malaysia 10-12 JULY
Denmark and Italy Trade-related intellectual property rights, access to medicines and human rights
 Summary Denmark and Italy Trade-related intellectual property rights, access to medicines and human rights October 2004 1. Denmark and Italy, as members of the European Union (EU), have committed themselves
Summary Denmark and Italy Trade-related intellectual property rights, access to medicines and human rights October 2004 1. Denmark and Italy, as members of the European Union (EU), have committed themselves
WORLD TRADE ORGANIZATION
 WORLD TRADE ORGANIZATION Council for Trade-Related Aspects of Intellectual Property Rights IP/C/W/424/Add.2 26 October 2004 (04-4530) Original: English TECHNICAL COOPERATION ACTIVITIES: INFORMATION FROM
WORLD TRADE ORGANIZATION Council for Trade-Related Aspects of Intellectual Property Rights IP/C/W/424/Add.2 26 October 2004 (04-4530) Original: English TECHNICAL COOPERATION ACTIVITIES: INFORMATION FROM
Trade-related intellectual property rights, trade in services and the fulfilment of children s rights - Botswana September 2004
 Trade-related intellectual property rights, trade in services and the fulfilment of children s rights - Botswana September 2004 Introduction 1. Botswana has emerged as a model of access to medicines and
Trade-related intellectual property rights, trade in services and the fulfilment of children s rights - Botswana September 2004 Introduction 1. Botswana has emerged as a model of access to medicines and
WORLD TRADE ORGANIZATION
 WORLD TRADE ORGANIZATION WT/L/412 3 September 2001 (01-4194) Original: English JOINT STATEMENT BY THE SAARC 1 COMMERCE MINISTERS ON THE FORTHCOMING FOURTH WTO MINISTERIAL CONFERENCE AT DOHA New Delhi,
WORLD TRADE ORGANIZATION WT/L/412 3 September 2001 (01-4194) Original: English JOINT STATEMENT BY THE SAARC 1 COMMERCE MINISTERS ON THE FORTHCOMING FOURTH WTO MINISTERIAL CONFERENCE AT DOHA New Delhi,
Answer of the Canadian National Group
 AIPPI INTERNATIONAL ASSOCIATION FOR THE PROTECTION OF INTELLECTUAL PROPERTY SPECIAL COMMITTEE Q94 QUESTIONNAIRE NO. 4 on the IMPLEMENTATION OF PARAGRAPH 6 OF THE DOHA DECLARATION ON TRIPS AND PUBLIC HEALTH
AIPPI INTERNATIONAL ASSOCIATION FOR THE PROTECTION OF INTELLECTUAL PROPERTY SPECIAL COMMITTEE Q94 QUESTIONNAIRE NO. 4 on the IMPLEMENTATION OF PARAGRAPH 6 OF THE DOHA DECLARATION ON TRIPS AND PUBLIC HEALTH
A I P P I ASSOCIATION INTERNATIONALE POUR LA PROTECTION DE LA PROPRIETE INTELLECTUELLE
 A I P P I ASSOCIATION INTERNATIONALE POUR LA PROTECTION DE LA PROPRIETE INTELLECTUELLE INTERNATIONAL ASSOCIATION FOR THE PROTECTION OF INTELLECTUAL PROPERTY INTERNATIONALE VEREINIGUNG FÜR DEN SCHUTZ DES
A I P P I ASSOCIATION INTERNATIONALE POUR LA PROTECTION DE LA PROPRIETE INTELLECTUELLE INTERNATIONAL ASSOCIATION FOR THE PROTECTION OF INTELLECTUAL PROPERTY INTERNATIONALE VEREINIGUNG FÜR DEN SCHUTZ DES
WORLD TRADE ORGANIZATION
 WORLD TRADE ORGANIZATION Council for Trade-Related Aspects of Intellectual Property Rights IP/C/63 26 November 2012 (12-6523) Original: English ANNUAL REVIEW OF THE DECISION ON THE IMPLEMENTATION OF PARAGRAPH
WORLD TRADE ORGANIZATION Council for Trade-Related Aspects of Intellectual Property Rights IP/C/63 26 November 2012 (12-6523) Original: English ANNUAL REVIEW OF THE DECISION ON THE IMPLEMENTATION OF PARAGRAPH
T H E W O R L D J O U R N A L O N J U R I S T I C P O L I T Y. BOLAR EXEMPTION VS. DATA EXCLUSIVITY: RIGHT TO HEALTH vs RIGHT OF PATENT HOLDER
 BOLAR EXEMPTION VS. DATA EXCLUSIVITY: RIGHT TO HEALTH vs RIGHT OF PATENT HOLDER Rhea Roy Mammen M.S. Ramaiah College of Law, Bangalore Introduction Pharmaceutical Patent has seen an increasing conflict
BOLAR EXEMPTION VS. DATA EXCLUSIVITY: RIGHT TO HEALTH vs RIGHT OF PATENT HOLDER Rhea Roy Mammen M.S. Ramaiah College of Law, Bangalore Introduction Pharmaceutical Patent has seen an increasing conflict
3. INTELLECTUAL PROPERTY POLICY & LEGAL FRAMEWORK
 3. INTELLECTUAL PROPERTY POLICY & LEGAL FRAMEWORK This section looks at the key issues and challenges related to the legal and policy framework in LDCs, before setting out a detailed checklist to guide
3. INTELLECTUAL PROPERTY POLICY & LEGAL FRAMEWORK This section looks at the key issues and challenges related to the legal and policy framework in LDCs, before setting out a detailed checklist to guide
BS5 Offering advocacy and member services The role of business associations in fostering Local Pharmaceutical Production
 Emmanuel Mujuru Deputy Chairman Southern African Generic Medicines Association (SAGMA) BS5 Offering advocacy and member services The role of business associations in fostering Local Pharmaceutical Production
Emmanuel Mujuru Deputy Chairman Southern African Generic Medicines Association (SAGMA) BS5 Offering advocacy and member services The role of business associations in fostering Local Pharmaceutical Production
TRIPs & Access to Medicines A choice between patents and patients! March 2010
 TRIPs & Access to Medicines A choice between patents and patients! March 2010 Every year, 14 million people in developing countries unnecessarily die of poverty-related and infectious diseases, such as
TRIPs & Access to Medicines A choice between patents and patients! March 2010 Every year, 14 million people in developing countries unnecessarily die of poverty-related and infectious diseases, such as
South-South Exchanges related to Patents in Developing Countries and LDCs: A Civil Society Reading
 South-South Exchanges related to Patents in Developing Countries and LDCs: A Civil Society Reading Heba Wanis BSc, MPH Researcher Egyptian Initiative for Personal Rights Second WIPO Inter-Regional Meeting
South-South Exchanges related to Patents in Developing Countries and LDCs: A Civil Society Reading Heba Wanis BSc, MPH Researcher Egyptian Initiative for Personal Rights Second WIPO Inter-Regional Meeting
The 4 th WTO Ministerial Conference and WTO Work Programme Emerging from Doha: An Assessment
 The 4 th WTO Ministerial Conference and WTO Work Programme Emerging from Doha: An Assessment According to the WTO a Ninth Round of Multilateral Trade Negotiations Launched According to the WTO on November
The 4 th WTO Ministerial Conference and WTO Work Programme Emerging from Doha: An Assessment According to the WTO a Ninth Round of Multilateral Trade Negotiations Launched According to the WTO on November
Trading Away Access to Medicines
 October 2009 Trading Away Access to Medicines How the European Commission s trade agenda has taken a wrong turn www.haiweb.org www.oxfam.org Summary Access to medicines poses a critical challenge in developing
October 2009 Trading Away Access to Medicines How the European Commission s trade agenda has taken a wrong turn www.haiweb.org www.oxfam.org Summary Access to medicines poses a critical challenge in developing
US-China Business Council Comments on the Draft Measures for the Compulsory Licensing of Patents
 US-China Business Council Comments on the Draft Measures for the Compulsory Licensing of Patents The US-China Business Council (USCBC) and its member companies appreciate the opportunity to submit comments
US-China Business Council Comments on the Draft Measures for the Compulsory Licensing of Patents The US-China Business Council (USCBC) and its member companies appreciate the opportunity to submit comments
IMPLICATION OF TRIPS AGREEMENT IN TANZANIA: CASE STUDY OF PHARMACEUTICAL PATENT AND HIV/ AIDS
 Ministry of Industry and Trade From the SelectedWorks of Johansein Rutaihwa Spring April, 2009 IMPLICATION OF TRIPS AGREEMENT IN TANZANIA: CASE STUDY OF PHARMACEUTICAL PATENT AND HIV/ AIDS Johansein L.
Ministry of Industry and Trade From the SelectedWorks of Johansein Rutaihwa Spring April, 2009 IMPLICATION OF TRIPS AGREEMENT IN TANZANIA: CASE STUDY OF PHARMACEUTICAL PATENT AND HIV/ AIDS Johansein L.
"Capacity-Building in the Face of the Emerging Challenges of Doha and the FTAA" 27 February 2002
 "Capacity-Building in the Face of the Emerging Challenges of Doha and the FTAA" 27 February 2002 THE CHALLENGES OF THE DOHA DEVELOPMENT AGENDA FOR LATIN AMERICAN AND CARIBBEAN COUNTRIES Inter-American
"Capacity-Building in the Face of the Emerging Challenges of Doha and the FTAA" 27 February 2002 THE CHALLENGES OF THE DOHA DEVELOPMENT AGENDA FOR LATIN AMERICAN AND CARIBBEAN COUNTRIES Inter-American
Citation. Kencho Palden (2009) Dangphu Dingphu: The Origin of the Bhutanese Folktales, Journal of Bhutan Studies, 21 (Winter 2009), pp
 Citation Kencho Palden (2009) Dangphu Dingphu: The Origin of the Bhutanese Folktales, Journal of Bhutan Studies, 21 (Winter 2009), pp.43-95. Intellectual Property, Access to Medicines and Public Health
Citation Kencho Palden (2009) Dangphu Dingphu: The Origin of the Bhutanese Folktales, Journal of Bhutan Studies, 21 (Winter 2009), pp.43-95. Intellectual Property, Access to Medicines and Public Health
CANCUN SESSION OF THE PARLIAMENTARY CONFERENCE ON THE WTO Cancún (Mexico), 9 and 12 September 2003
 CANCUN SESSION OF THE PARLIAMENTARY CONFERENCE ON THE WTO Cancún (Mexico), 9 and 12 September 2003 Organised jointly by the Inter-Parliamentary Union and the European Parliament with the support of the
CANCUN SESSION OF THE PARLIAMENTARY CONFERENCE ON THE WTO Cancún (Mexico), 9 and 12 September 2003 Organised jointly by the Inter-Parliamentary Union and the European Parliament with the support of the
Anti-counterfeiting laws and access to essential medicines in East and Southern Africa
 Regional Network for Equity in Health in East and Southern Africa (EQUINET) Anti-counterfeiting laws and access to essential medicines in East and Southern Africa EQUINET Policy Brief number 22 Co-published
Regional Network for Equity in Health in East and Southern Africa (EQUINET) Anti-counterfeiting laws and access to essential medicines in East and Southern Africa EQUINET Policy Brief number 22 Co-published
POVERTY, TRADE AND HEALTH: AN EMERGING HEALTH DEVELOPMENT ISSUE. Report of the Regional Director EXECUTIVE SUMMARY
 17 June 2006 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-sixth session Addis Ababa, Ethiopia, 28 August 1 September 2006 Provisional agenda item 8.3 POVERTY, TRADE AND HEALTH: AN EMERGING HEALTH
17 June 2006 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-sixth session Addis Ababa, Ethiopia, 28 August 1 September 2006 Provisional agenda item 8.3 POVERTY, TRADE AND HEALTH: AN EMERGING HEALTH
International Gender and Trade Network Monthly Bulletin (IGTN) Vol. 2 No. 10 December 2002 TRIPS and Pubic Health
 International Gender and Trade Network Monthly Bulletin (IGTN) Vol. 2 No. 10 December 2002 TRIPS and Pubic Health TRIPS and Public Health - Controversy Over the TRIPS and Public Health Agreement - Advocacy
International Gender and Trade Network Monthly Bulletin (IGTN) Vol. 2 No. 10 December 2002 TRIPS and Pubic Health TRIPS and Public Health - Controversy Over the TRIPS and Public Health Agreement - Advocacy
Intellectual Property Department Hong Kong, China. Contents
 Intellectual Property Department Hong Kong, China Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 3 Section 3: Experimental use and/or scientific research... 3 Section
Intellectual Property Department Hong Kong, China Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 3 Section 3: Experimental use and/or scientific research... 3 Section
English summary of book L OMS en péril» (WHO in peril) in French, by the author, Yves Beigbeder 1.
 EXECUTIVE SUMMARY English summary of book L OMS en péril» (WHO in peril) in French, by the author, Yves Beigbeder 1. In his Foreword, Dr German Velasquez (Senior Consultant for health and development,
EXECUTIVE SUMMARY English summary of book L OMS en péril» (WHO in peril) in French, by the author, Yves Beigbeder 1. In his Foreword, Dr German Velasquez (Senior Consultant for health and development,
LL.M. in International Legal Studies WTO LAW
 LL.M. in International Legal Studies WTO LAW Prof. Dr. Friedl WEISS Institute for European, International and Comparative Law - University of Vienna Winter Semester 2012/13 Part II History & Institutions
LL.M. in International Legal Studies WTO LAW Prof. Dr. Friedl WEISS Institute for European, International and Comparative Law - University of Vienna Winter Semester 2012/13 Part II History & Institutions
A COMPARATIVE STUDY OF FOREIGN INVESTMENT REGULATIONS IN INDIA AND MAJOR WORLD ECONOMIES
 A COMPARATIVE STUDY OF FOREIGN INVESTMENT REGULATIONS IN INDIA AND MAJOR WORLD ECONOMIES Ms. Dhanya. J. S Assistant Professor,MBA Department,CET School Of Management,Trivandrum, Kerala ----------------------------------------------------------------------------------------------------------------------------------
A COMPARATIVE STUDY OF FOREIGN INVESTMENT REGULATIONS IN INDIA AND MAJOR WORLD ECONOMIES Ms. Dhanya. J. S Assistant Professor,MBA Department,CET School Of Management,Trivandrum, Kerala ----------------------------------------------------------------------------------------------------------------------------------
Facilitation: Basics on drug development and registration process. 9:15 Political History of Patent Law Globalization
 Human Rights and Access to Medicines 12 16 May University of Pretoria, South Africa Monday and Thursday Room 1-5.1, Law Bulding Tuesday, Wednesday and Friday Room 2-2.1, Law Building Mr Sean Flynn, Washington
Human Rights and Access to Medicines 12 16 May University of Pretoria, South Africa Monday and Thursday Room 1-5.1, Law Bulding Tuesday, Wednesday and Friday Room 2-2.1, Law Building Mr Sean Flynn, Washington
Three-Pronged Strategy to Address Refugee Urban Health: Advocate, Support and Monitor
 Urban Refugee Health 1. The issue Many of the health strategies, policies and interventions for refugees are based on past experiences where refugees are situated in camp settings and in poor countries.
Urban Refugee Health 1. The issue Many of the health strategies, policies and interventions for refugees are based on past experiences where refugees are situated in camp settings and in poor countries.
IMPLEMENTATION OF THE WTO DECISION ON COMPULSORY LICENSING FOR COUNTRIES WITH INSUFFICIENT OR NO MANUFACTURING CAPACITY IN THE PHARMACEUTICAL SECTOR
 BRIEFING PAPER Policy Department External Policies IMPLEMENTATION OF THE WTO DECISION ON COMPULSORY LICENSING FOR COUNTRIES WITH INSUFFICIENT OR NO MANUFACTURING CAPACITY IN THE PHARMACEUTICAL SECTOR INTERNATIONAL
BRIEFING PAPER Policy Department External Policies IMPLEMENTATION OF THE WTO DECISION ON COMPULSORY LICENSING FOR COUNTRIES WITH INSUFFICIENT OR NO MANUFACTURING CAPACITY IN THE PHARMACEUTICAL SECTOR INTERNATIONAL
Informal Brief. The Treatment Of Intellectual Property In The Ministerial Declaration: Mandated Negotiations And Reviews
 Informal Brief The Treatment Of Intellectual Property In The Ministerial Declaration: Mandated Negotiations And Reviews By David Vivas Eugui Senior Attorney, Center for International Environmental Law
Informal Brief The Treatment Of Intellectual Property In The Ministerial Declaration: Mandated Negotiations And Reviews By David Vivas Eugui Senior Attorney, Center for International Environmental Law
TITLE: PATRICIA ANNE D. STA. MARIA AUTHOR: DATE: 2015 ABSTRACT:
 TITLE: LIFE, DEATH, AND DATA: EXAMINING THE HUMAN RIGHTS IMPLICATIONS OF INTRODUCING DATA EXCLUSIVITY TO INDIA'S PHARMACEUTICAL SYSTEM, IN LIGHT OF THE GLOBAL SITUATION OF DISEASES SUCH AS HIV/AIDS IN
TITLE: LIFE, DEATH, AND DATA: EXAMINING THE HUMAN RIGHTS IMPLICATIONS OF INTRODUCING DATA EXCLUSIVITY TO INDIA'S PHARMACEUTICAL SYSTEM, IN LIGHT OF THE GLOBAL SITUATION OF DISEASES SUCH AS HIV/AIDS IN
The Doha Development Agenda: Reflections on the Road Ahead
 Asia-Pacific Review, Vol. 9, No. 1, 2002 The Doha Development Agenda: Reflections on the Road Ahead MIKE MOORE From 9 to 14 November 2001, Qatar hosted the Fourth Ministerial Conference of the World Trade
Asia-Pacific Review, Vol. 9, No. 1, 2002 The Doha Development Agenda: Reflections on the Road Ahead MIKE MOORE From 9 to 14 November 2001, Qatar hosted the Fourth Ministerial Conference of the World Trade
INTERVIEW WITH JAMES LOVE ON HOW PEOPLE CAN GET INVOLVED
 INTERVIEW WITH JAMES LOVE ON HOW PEOPLE CAN GET INVOLVED James Packard Jamie Love is the founder and director of Knowledge Ecology International (KEI), a Washington- and Geneva-based non-governmental organization
INTERVIEW WITH JAMES LOVE ON HOW PEOPLE CAN GET INVOLVED James Packard Jamie Love is the founder and director of Knowledge Ecology International (KEI), a Washington- and Geneva-based non-governmental organization
MODULE. Conclusion. ESTIMATED TIME: 3 hours
 MODULE 11 Conclusion ESTIMATED TIME: 3 hours 1 Overview I. MODULE 1 INTRODUCTION TO THE WTO SUMMARY... 3 II. MODULE 2 INTRODUCTION TO THE TRIPS AGREEMENT SUMMARY... 5 III. MODULE 3 COPYRIGHT AND RELATED
MODULE 11 Conclusion ESTIMATED TIME: 3 hours 1 Overview I. MODULE 1 INTRODUCTION TO THE WTO SUMMARY... 3 II. MODULE 2 INTRODUCTION TO THE TRIPS AGREEMENT SUMMARY... 5 III. MODULE 3 COPYRIGHT AND RELATED
Trade liberalisation and globalisation: What are the impacts on women's lives?
 Trade liberalisation and globalisation: What are the impacts on women's lives? European Women's Lobby Barcelona, 9 June 2001 To kick off our discussions today I would like to refer to the perspectives
Trade liberalisation and globalisation: What are the impacts on women's lives? European Women's Lobby Barcelona, 9 June 2001 To kick off our discussions today I would like to refer to the perspectives
Report on the WTO Ministerial Conference in Seattle. November 30 to December 3, by Dr. Martin J. LUTZ (Switzerland) Chairman Q 94 - GATT/WTO
 REPORTS Report on the WTO Ministerial Conference in Seattle November 30 to December 3, 1999 by Dr. Martin J. LUTZ (Switzerland) Chairman Q 94 - GATT/WTO A The Set-up WTO called for a Ministerial Conference
REPORTS Report on the WTO Ministerial Conference in Seattle November 30 to December 3, 1999 by Dr. Martin J. LUTZ (Switzerland) Chairman Q 94 - GATT/WTO A The Set-up WTO called for a Ministerial Conference
Draft 2 Hanoi, 2006 DECREE
 THE GOVERNMENT No. /2006/ND - CP THE SOCIALIST REPUBLIC OF VIETNAM Independence Freedom Happiness ------------------------------ Draft 2 Hanoi, 2006 DECREE Making detailed provisions and providing guidelines
THE GOVERNMENT No. /2006/ND - CP THE SOCIALIST REPUBLIC OF VIETNAM Independence Freedom Happiness ------------------------------ Draft 2 Hanoi, 2006 DECREE Making detailed provisions and providing guidelines
THE IMPLICATIONS OF RWANDA S PARAGRAPH 6 AGREEMENT
 THE IMPLICATIONS OF RWANDA S PARAGRAPH 6 AGREEMENT WITH CANADA FOR OTHER DEVELOPING COUNTRIES Christina Cotter I. Introduction On July 17, 2007, Rwanda became the first country to notify the World Trade
THE IMPLICATIONS OF RWANDA S PARAGRAPH 6 AGREEMENT WITH CANADA FOR OTHER DEVELOPING COUNTRIES Christina Cotter I. Introduction On July 17, 2007, Rwanda became the first country to notify the World Trade
Assessing Barriers to Trade in Education Services in Developing ESCAP Countries: An Empirical Exercise WTO/ARTNeT Short-term Research Project
 Assessing Barriers to Trade in Education Services in Developing ESCAP Countries: An Empirical Exercise WTO/ARTNeT Short-term Research Project Ajitava Raychaudhuri, Jadavpur University Kolkata, India And
Assessing Barriers to Trade in Education Services in Developing ESCAP Countries: An Empirical Exercise WTO/ARTNeT Short-term Research Project Ajitava Raychaudhuri, Jadavpur University Kolkata, India And
WIPO Sub-Regional Workshop on Patent Policy and its Legislative Implementation
 WIPO Sub-Regional Workshop on Patent Policy and its Legislative Implementation Topic 12: Patent-related provisions in the framework of preferential trade agreements Marco M. ALEMAN Deputy Director, Patent
WIPO Sub-Regional Workshop on Patent Policy and its Legislative Implementation Topic 12: Patent-related provisions in the framework of preferential trade agreements Marco M. ALEMAN Deputy Director, Patent
Patent Act, B.E (1979) As Amended until Patent Act (No.3), B.E (1999) Translation
 Patent Act, B.E. 2522 (1979) As Amended until Patent Act (No.3), B.E. 2542 (1999) Translation BHUMIBOL ADULYADEJ, REX. Given on the 11th day of March, B.E. 2522; Being the 34th year of the present Reign
Patent Act, B.E. 2522 (1979) As Amended until Patent Act (No.3), B.E. 2542 (1999) Translation BHUMIBOL ADULYADEJ, REX. Given on the 11th day of March, B.E. 2522; Being the 34th year of the present Reign
Proposal for a COUNCIL DECISION
 EUROPEAN COMMISSION Brussels, 27.7.2018 COM(2018) 350 final 2018/0214 (NLE) Proposal for a COUNCIL DECISION on the accession of the European Union to the Geneva Act of the Lisbon Agreement on Appellations
EUROPEAN COMMISSION Brussels, 27.7.2018 COM(2018) 350 final 2018/0214 (NLE) Proposal for a COUNCIL DECISION on the accession of the European Union to the Geneva Act of the Lisbon Agreement on Appellations
Executive summary. Transparency International
 Executive summary Transparency International Every year, the world spends more than US $3 trillion on health services, most of which is financed by taxpayers. These large flows of funds are an attractive
Executive summary Transparency International Every year, the world spends more than US $3 trillion on health services, most of which is financed by taxpayers. These large flows of funds are an attractive
Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law
 !!! Dangers for Access to Medicines in the Trans-Pacific Partnership Agreement: Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law ! Issue US TPPA Proposal Andean Community
!!! Dangers for Access to Medicines in the Trans-Pacific Partnership Agreement: Comparative Analysis of the U.S. Intellectual Property Proposal and Peruvian Law ! Issue US TPPA Proposal Andean Community
PRESENTATION ON KENYA S EXPERIENCE AT THE WTO
 PRESENTATION ON KENYA S EXPERIENCE AT THE WTO PRESENTATION BY: AMB. NELSON NDIRANGU DIRECTOR ECONOMIC AFFAIRS AND COMMERCIAL DIPLOMACY DIRECTORATE MINISTRY OF FOREIGN AFFAIRS 28 TH AUGUST 2017 OUTLINE
PRESENTATION ON KENYA S EXPERIENCE AT THE WTO PRESENTATION BY: AMB. NELSON NDIRANGU DIRECTOR ECONOMIC AFFAIRS AND COMMERCIAL DIPLOMACY DIRECTORATE MINISTRY OF FOREIGN AFFAIRS 28 TH AUGUST 2017 OUTLINE
DRAFT REPORT. EN United in diversity EN 2012/2135(INI)
 EUROPEAN PARLIAMT 2009-2014 Committee on Development 25.7.2012 2012/2135(INI) DRAFT REPORT on development aspects of intellectual property rights on genetic resources: the impact on poverty reduction in
EUROPEAN PARLIAMT 2009-2014 Committee on Development 25.7.2012 2012/2135(INI) DRAFT REPORT on development aspects of intellectual property rights on genetic resources: the impact on poverty reduction in
COMMENTARY I. Comment: access to essential medicines promoting human rights over free trade and intellectual property claims
 COMMENTARY I Comment: access to essential medicines promoting human rights over free trade and intellectual property claims HEINZ KLUG* 1. Introduction Over the past five years, there has been an intense
COMMENTARY I Comment: access to essential medicines promoting human rights over free trade and intellectual property claims HEINZ KLUG* 1. Introduction Over the past five years, there has been an intense
IJRIM Volume 2, Issue 6 (June 2012) (ISSN ) WORLD TRADE ORGANIZATION: ITS IMPACT ON INDIAN ECONOMY ABSTRACT
 WORLD TRADE ORGANIZATION: ITS IMPACT ON INDIAN ECONOMY Neeraj Dalal* ABSTRACT The birth of World Trade Organization (WTO) Came into existence on January 1, 1995 holds a great promise for the entire world
WORLD TRADE ORGANIZATION: ITS IMPACT ON INDIAN ECONOMY Neeraj Dalal* ABSTRACT The birth of World Trade Organization (WTO) Came into existence on January 1, 1995 holds a great promise for the entire world
Response to the EC consultation on the future direction of EU trade policy. 28 July 2010
 Response to the EC consultation on the future direction of EU trade policy 28 July 2010 Question 1: Now that the new Lisbon Treaty has entered into force, how can we best ensure that our future trade policy
Response to the EC consultation on the future direction of EU trade policy 28 July 2010 Question 1: Now that the new Lisbon Treaty has entered into force, how can we best ensure that our future trade policy
Making the WTO More Supportive of Development. How to help developing countries integrate into the global trading system.
 Car trailer-trucks in Brazil Making the WTO More Supportive of Development Bernard Hoekman How to help developing countries integrate into the global trading system IN WORLD trade negotiations there is
Car trailer-trucks in Brazil Making the WTO More Supportive of Development Bernard Hoekman How to help developing countries integrate into the global trading system IN WORLD trade negotiations there is
Médecins Sans Frontières response to : EC Issue Paper: The EU role in global health Consultation document including questions to be answered
 Médecins Sans Frontières response to : EC Issue Paper: The EU role in global health Consultation document including questions to be answered Médecins Sans Frontières (MSF) is an international, medical
Médecins Sans Frontières response to : EC Issue Paper: The EU role in global health Consultation document including questions to be answered Médecins Sans Frontières (MSF) is an international, medical
PROVISIONAL SUMMARY RECORD OF THE ELEVENTH MEETING
 3 EXECUTIVE BOARD EB140/PSR/11 140th session 3 March 2017 PROVISIONAL SUMMARY RECORD OF THE ELEVENTH MEETING WHO headquarters, Geneva Friday, 27 January 2017, scheduled at 14:30 Chairman: Dr R. BUSUTTIL
3 EXECUTIVE BOARD EB140/PSR/11 140th session 3 March 2017 PROVISIONAL SUMMARY RECORD OF THE ELEVENTH MEETING WHO headquarters, Geneva Friday, 27 January 2017, scheduled at 14:30 Chairman: Dr R. BUSUTTIL
The Trans-Pacific Partnership
 The Trans-Pacific Partnership A Side-By-Side Comparison with: Comparison Vol. 3 (Rev.) The United States - Colombia Trade Promotion Agreement of 2012 The United States - Korea Free Trade Agreement of 2012
The Trans-Pacific Partnership A Side-By-Side Comparison with: Comparison Vol. 3 (Rev.) The United States - Colombia Trade Promotion Agreement of 2012 The United States - Korea Free Trade Agreement of 2012
PROGRAMME FOR CHINA-AFRICA COOPERATION IN ECONOMIC AND SOCIAL DEVELOPMENT
 PROGRAMME FOR CHINA-AFRICA COOPERATION IN ECONOMIC AND SOCIAL DEVELOPMENT The Forum on China-Africa Co-operation - Ministerial Conference 2000 was held in Beijing, China from 10 to 12 October 2000. Ministers
PROGRAMME FOR CHINA-AFRICA COOPERATION IN ECONOMIC AND SOCIAL DEVELOPMENT The Forum on China-Africa Co-operation - Ministerial Conference 2000 was held in Beijing, China from 10 to 12 October 2000. Ministers
EURO-LATIN AMERICAN PARLIAMENTARY ASSEMBLY. Committee for Economic, Financial and Commercial Affairs WORKING DOCUMENT
 Euro-Latin American Parliamentary Assembly Assemblée Parlementaire Euro-Latino Américaine Asamblea Parlamentaria Euro-Latinoamericana Assembleia ParlamentarEuro-Latino-Americana EURO-LATIN AMERICAN PARLIAMTARY
Euro-Latin American Parliamentary Assembly Assemblée Parlementaire Euro-Latino Américaine Asamblea Parlamentaria Euro-Latinoamericana Assembleia ParlamentarEuro-Latino-Americana EURO-LATIN AMERICAN PARLIAMTARY
Letter dated 29 October 2003 from the Permanent Representative of Denmark to the United Nations addressed to the Secretary-General
 United Nations General Assembly Distr.: General 30 October 2003 Original: English A/58/542 Fifty-eighth session Agenda item 104 (b) Follow-up to the International Conference on Financing for Development:
United Nations General Assembly Distr.: General 30 October 2003 Original: English A/58/542 Fifty-eighth session Agenda item 104 (b) Follow-up to the International Conference on Financing for Development:
Public Health Association of Australia: Policy-at-a-glance Trade Agreements & Health Policy
 Public Health Association of Australia: Policy-at-a-glance Trade Agreements & Health Policy Key message: 1. Trade agreements should not limit or override a nation s ability to foster and maintain systems
Public Health Association of Australia: Policy-at-a-glance Trade Agreements & Health Policy Key message: 1. Trade agreements should not limit or override a nation s ability to foster and maintain systems
Course on WTO Law and Jurisprudence Part III: WTO Dispute Settlement Procedures. Which legal instruments can be invoked in a WTO dispute?
 Course on WTO Law and Jurisprudence Part III: WTO Dispute Settlement Procedures Which legal instruments can be invoked in a WTO dispute? Session 5 2 November 2017 AGENDA a) What instruments can be invoked
Course on WTO Law and Jurisprudence Part III: WTO Dispute Settlement Procedures Which legal instruments can be invoked in a WTO dispute? Session 5 2 November 2017 AGENDA a) What instruments can be invoked
World Trade Organisation Agreements: Implications for equity and health in Southern Africa
 World Trade Organisation Agreements: Implications for equity and health in Southern Africa G Munot Regional Network for Equity in Health in Southern Africa (EQUINET) with the Southern African Development
World Trade Organisation Agreements: Implications for equity and health in Southern Africa G Munot Regional Network for Equity in Health in Southern Africa (EQUINET) with the Southern African Development
Having regard to the Treaty establishing the European Economic Community, and in particular Article 100 thereof;
 DIRECTIVE 75/319/EEC Council Directive 75/319/EEC of 20 May 1975 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (OJ No L 147 of
DIRECTIVE 75/319/EEC Council Directive 75/319/EEC of 20 May 1975 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (OJ No L 147 of
EU Trade Policy and IPRs Generally, all EU external economic policies including trade policies are first drafted and considered by the European Commis
 17 FTA policy- Making in the EU and its Effects : Policies on Geographic Indicators and Medicines/Medical Equipment (*) Overseas Researcher: Momoko NISHIMURA (**) Recently, the European Union has shifted
17 FTA policy- Making in the EU and its Effects : Policies on Geographic Indicators and Medicines/Medical Equipment (*) Overseas Researcher: Momoko NISHIMURA (**) Recently, the European Union has shifted
Regional Comprehensive Economic Partnership. Intellectual Property Chapter and the Impact on Access to Medicines
 MSF RCEP IP Chapter Technical Analysis NOVEMBER 2016 Regional Comprehensive Economic Partnership Intellectual Property Chapter and the Impact on Access to Medicines This briefing note details Médecins
MSF RCEP IP Chapter Technical Analysis NOVEMBER 2016 Regional Comprehensive Economic Partnership Intellectual Property Chapter and the Impact on Access to Medicines This briefing note details Médecins
8 LEGAL METROLOGY IN 2020 ROLE OF GOVERNMENTS OF AFRICA S DEVELOPING COUNTRIES Jackai Derrick Mosima, Department of Prices and Metrology, Cameroon
 8 LEGAL METROLOGY IN 2020 ROLE OF GOVERNMENTS OF AFRICA S DEVELOPING COUNTRIES Jackai Derrick Mosima, Department of Prices and Metrology, Cameroon Introduction In Africa, as in every other society, weights
8 LEGAL METROLOGY IN 2020 ROLE OF GOVERNMENTS OF AFRICA S DEVELOPING COUNTRIES Jackai Derrick Mosima, Department of Prices and Metrology, Cameroon Introduction In Africa, as in every other society, weights
COMMUNICATION FROM THE COMMISSION TO THE COUNCIL, THE EUROPEAN PARLIAMENT, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
 EN EN EN EUROPEAN COMMISSION Brussels, 31.3.2010 COM(2010)128 final COMMUNICATION FROM THE COMMISSION TO THE COUNCIL, THE EUROPEAN PARLIAMENT, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE
EN EN EN EUROPEAN COMMISSION Brussels, 31.3.2010 COM(2010)128 final COMMUNICATION FROM THE COMMISSION TO THE COUNCIL, THE EUROPEAN PARLIAMENT, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE
Business and the global economy
 International Chamber of Commerce The world business organization Business and the global economy ICC statement on behalf of world business to the Heads of State and Government attending the Evian Summit,
International Chamber of Commerce The world business organization Business and the global economy ICC statement on behalf of world business to the Heads of State and Government attending the Evian Summit,
Trade in Services: A South Asian Agenda NITYA NANDA
 Trade in Services: A South Asian Agenda 1 NITYA NANDA Outline of the Presentation 2 Trade in Services: Some questions Some myths and realities GATS commitments Meaning of GATS commitments Assessment of
Trade in Services: A South Asian Agenda 1 NITYA NANDA Outline of the Presentation 2 Trade in Services: Some questions Some myths and realities GATS commitments Meaning of GATS commitments Assessment of
Notwithstanding Article 29, any invention that is liable to injure public order, morality or public health shall not be patented (Article 32).
 Japan Patent Office (JPO) Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 2 Section 3: Experimental use and/or scientific research... 3 Section 4: Preparation of medicines...
Japan Patent Office (JPO) Contents Section 1: General... 1 Section 2: Private and/or non-commercial use... 2 Section 3: Experimental use and/or scientific research... 3 Section 4: Preparation of medicines...
WORLD TRADE ORGANIZATION
 WORLD TRADE ORGANIZATION WT/DS136/11 28 February 2001 (01-0980) UNITED STATES ANTI-DUMPING ACT OF 1916 Arbitration under Article 21.3(c) of the Understanding on Rules and Procedures Governing the Settlement
WORLD TRADE ORGANIZATION WT/DS136/11 28 February 2001 (01-0980) UNITED STATES ANTI-DUMPING ACT OF 1916 Arbitration under Article 21.3(c) of the Understanding on Rules and Procedures Governing the Settlement
INDIAN PATENTS. Request for Examination. 48 months from priority*
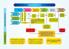 INDIAN PATENTS INDIAN PATENT PROSECUTION ASA FACILITATION Direct filing (Priority application) 31 months from Priority (PCT Route) Filing 12 months from Basic Application (Convention Route) 18 months from
INDIAN PATENTS INDIAN PATENT PROSECUTION ASA FACILITATION Direct filing (Priority application) 31 months from Priority (PCT Route) Filing 12 months from Basic Application (Convention Route) 18 months from
Presented at the Closing Plenary Session on 11 September 2006
 AEBF10 CHAIRMAN S STATEMENT 11 September 2006 The 10 th Asia-Europe Business Forum (AEBF10), Helsinki Chairman s Statement Presented at the Closing Plenary Session on 11 September 2006 The 10 th Asia-Europe
AEBF10 CHAIRMAN S STATEMENT 11 September 2006 The 10 th Asia-Europe Business Forum (AEBF10), Helsinki Chairman s Statement Presented at the Closing Plenary Session on 11 September 2006 The 10 th Asia-Europe
ACCESSION KIT: THE MADRID SYSTEM FOR THE INTERNATIONAL REGISTRATION OF MARKS
 ACCESSION KIT: THE MADRID SYSTEM FOR THE INTERNATIONAL REGISTRATION OF MARKS Contents THE MADRID SYSTEM FOR THE INTERNATIONAL REGISTRATION OF MARKS... 1 ADVANTAGES OF THE MADRID SYSTEM... 1 BENEFITS FOR
ACCESSION KIT: THE MADRID SYSTEM FOR THE INTERNATIONAL REGISTRATION OF MARKS Contents THE MADRID SYSTEM FOR THE INTERNATIONAL REGISTRATION OF MARKS... 1 ADVANTAGES OF THE MADRID SYSTEM... 1 BENEFITS FOR
IS ARTICLE 31BIS ENOUGH? THE NEED TO PROMOTE ECONOMIES OF SCALE IN THE INTERNATIONAL COMPULSORY LICENSING SYSTEM
 IS ARTICLE 31BIS ENOUGH? THE NEED TO PROMOTE ECONOMIES OF SCALE IN THE INTERNATIONAL COMPULSORY LICENSING SYSTEM I. INTRODUCTION Every year, millions of people die from diseases that ravage populations
IS ARTICLE 31BIS ENOUGH? THE NEED TO PROMOTE ECONOMIES OF SCALE IN THE INTERNATIONAL COMPULSORY LICENSING SYSTEM I. INTRODUCTION Every year, millions of people die from diseases that ravage populations
The Beijing Declaration on South-South Cooperation for Child Rights in the Asia Pacific Region
 The Beijing Declaration on South-South Cooperation for Child Rights in the Asia Pacific Region 1. We, the delegations of Afghanistan, Bangladesh, Bhutan, Brunei Darussalam, Cambodia, China, Democratic
The Beijing Declaration on South-South Cooperation for Child Rights in the Asia Pacific Region 1. We, the delegations of Afghanistan, Bangladesh, Bhutan, Brunei Darussalam, Cambodia, China, Democratic
Public WTO Trade Facilitation - Improvements to GATT Article VIII on Fees and Formalities Connected with Importation and Exportation
 Public 11.07.2002 WTO Trade Facilitation - Improvements to GATT Article VIII on Fees and Formalities Connected with Importation and Exportation 1 Draft Submission from the European Communities Introduction
Public 11.07.2002 WTO Trade Facilitation - Improvements to GATT Article VIII on Fees and Formalities Connected with Importation and Exportation 1 Draft Submission from the European Communities Introduction
The Medicines (Traditional Herbal Medicinal Products for Human Use) Regulations 2005
 STATUTORY INSTRUMENTS 2005 No. 2750 MEDICINES The Medicines (Traditional Herbal Medicinal Products for Human Use) Regulations 2005 Made - - - - - 6th October 2005 Laid before Parliament 7th October 2005
STATUTORY INSTRUMENTS 2005 No. 2750 MEDICINES The Medicines (Traditional Herbal Medicinal Products for Human Use) Regulations 2005 Made - - - - - 6th October 2005 Laid before Parliament 7th October 2005
Health services trade
 Health Services Trade: How Thailand may benefit from trade liberalisation with ASEAN Jutamas Arunanondchai FPRI 4th AEF Meeting, 22-23 June, 2004. (This version 21/06/04) 1 Health services trade Scope
Health Services Trade: How Thailand may benefit from trade liberalisation with ASEAN Jutamas Arunanondchai FPRI 4th AEF Meeting, 22-23 June, 2004. (This version 21/06/04) 1 Health services trade Scope
ITUC 1 Contribution to the pre-conference negotiating text for the UNCTAD XII Conference in Accra, April
 ITUC 1 Contribution to the pre-conference negotiating text for the UNCTAD XII Conference in Accra, 20-25 April 2008 2 Introduction: Trade, Employment and Inequality 1. The ITUC welcomes this opportunity
ITUC 1 Contribution to the pre-conference negotiating text for the UNCTAD XII Conference in Accra, 20-25 April 2008 2 Introduction: Trade, Employment and Inequality 1. The ITUC welcomes this opportunity
World business and the multilateral trading system
 International Chamber of Commerce The world business organization Policy statement Commission on Trade and Investment Policy World business and the multilateral trading system ICC policy recommendations
International Chamber of Commerce The world business organization Policy statement Commission on Trade and Investment Policy World business and the multilateral trading system ICC policy recommendations
Private Actors Involvement in International Public Policymaking
 Private Actors Involvement in International Public Policymaking Corina Grigore MPA Programme London School of Economics and Political Science c.m.grigore@lse.ac.uk Georgeta Nae Faculty of Economics and
Private Actors Involvement in International Public Policymaking Corina Grigore MPA Programme London School of Economics and Political Science c.m.grigore@lse.ac.uk Georgeta Nae Faculty of Economics and
RIS. in collaboration with MINISTRY OF INDIAN COUNCIL OF ENVIRONMENT & FORESTS MEDICAL RESEARCH
 RIS in collaboration with MINISTRY OF INDIAN COUNCIL OF ENVIRONMENT & FORESTS MEDICAL RESEARCH International Conference on Access and Benefit Sharing for Genetic Resources March 6-7, 2008, Magnolia Hall,
RIS in collaboration with MINISTRY OF INDIAN COUNCIL OF ENVIRONMENT & FORESTS MEDICAL RESEARCH International Conference on Access and Benefit Sharing for Genetic Resources March 6-7, 2008, Magnolia Hall,
INTERNATIONALLY RECOGNISED CORE LABOUR STANDARDS IN BARBADOS
 INTERNATIONAL TRADE UNION CONFEDERATION (ITUC) INTERNATIONALLY RECOGNISED CORE LABOUR STANDARDS IN BARBADOS REPORT FOR THE WTO GENERAL COUNCIL REVIEW OF THE TRADE POLICIES OF BARBADOS (Geneva, 17 and 19
INTERNATIONAL TRADE UNION CONFEDERATION (ITUC) INTERNATIONALLY RECOGNISED CORE LABOUR STANDARDS IN BARBADOS REPORT FOR THE WTO GENERAL COUNCIL REVIEW OF THE TRADE POLICIES OF BARBADOS (Geneva, 17 and 19
Issue Brief The Doha WTO Ministerial
 Nathan Associates Inc. Issue Brief The Doha WTO Ministerial OVERVIEW OF DEVELOPING COUNTRY CONCERNS Developing countries have become an increasingly vocal, and increasingly powerful, force in multilateral
Nathan Associates Inc. Issue Brief The Doha WTO Ministerial OVERVIEW OF DEVELOPING COUNTRY CONCERNS Developing countries have become an increasingly vocal, and increasingly powerful, force in multilateral
Ericsson Position on Questionnaire on the Future Patent System in Europe
 Ericsson Position on Questionnaire on the Future Patent System in Europe Executive Summary Ericsson welcomes the efforts of the European Commission to survey the patent systems in Europe in order to see
Ericsson Position on Questionnaire on the Future Patent System in Europe Executive Summary Ericsson welcomes the efforts of the European Commission to survey the patent systems in Europe in order to see
BRICS: A CALL TO ACTION
 BRICS: A CALL TO ACTION How the BRICS Countries Can Help End Neglected Tropical Diseases In July 2014, heads of state and senior ministerial officials from Brazil, Russia, India, China and South Africa
BRICS: A CALL TO ACTION How the BRICS Countries Can Help End Neglected Tropical Diseases In July 2014, heads of state and senior ministerial officials from Brazil, Russia, India, China and South Africa
IPP278 v.1 rev. Cambodia - Second Health Sector Support Project (HSSP2) Indigenous Peoples Planning Framework (IPPF)
 Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized IPP278 v.1 rev. Cambodia - Second Health Sector Support Project (HSSP2) Indigenous Peoples
Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized IPP278 v.1 rev. Cambodia - Second Health Sector Support Project (HSSP2) Indigenous Peoples
Global Access to Medicines Program Compiled by Stephanie Rosenberg. December 2, This chart compares provisions from the following texts:
 Comparative chart of patent and data provisions in the TRIPS, Free Trade s between Trans-Pacific negotiating countries and the U.S., and the U.S. proposal to the Trans-Pacific This chart compares provisions
Comparative chart of patent and data provisions in the TRIPS, Free Trade s between Trans-Pacific negotiating countries and the U.S., and the U.S. proposal to the Trans-Pacific This chart compares provisions
SC: Welfare panels can t evaluate dowry complaints
 Contact: 097418 69722 Website: www.navodayafoundation.in, www.yesupsc.com 15 September 2018 Daily News Pedia SC: Welfare panels can t evaluate dowry complaints Supreme Court judgement to scrap family welfare
Contact: 097418 69722 Website: www.navodayafoundation.in, www.yesupsc.com 15 September 2018 Daily News Pedia SC: Welfare panels can t evaluate dowry complaints Supreme Court judgement to scrap family welfare
Global Diseases, Global Patents and Differential Treatment in WTO Law
 Global Diseases, Global Patents and Differential Treatment in WTO Law Bradly Condon, LLB, LLM, PhD, Professor of International Trade Law, ITAM and Senior Fellow, Tim Fischer Centre for Global Trade and
Global Diseases, Global Patents and Differential Treatment in WTO Law Bradly Condon, LLB, LLM, PhD, Professor of International Trade Law, ITAM and Senior Fellow, Tim Fischer Centre for Global Trade and
INDIAN ECONOMY CURRENT AFFAIRS 2017 NATIONAL IPR POLICY, 2016
 INDIAN ECONOMY CURRENT AFFAIRS 2017 NATIONAL IPR POLICY, 2016 Intellectual property (IP) refers to creations of the mind, such as inventions, literary and artistic works, designs and symbols and names
INDIAN ECONOMY CURRENT AFFAIRS 2017 NATIONAL IPR POLICY, 2016 Intellectual property (IP) refers to creations of the mind, such as inventions, literary and artistic works, designs and symbols and names
Multilateral Trading System in 2013 The Current State of Affairs & Expectations for the Short Term Bipul Chatterjee
 Multilateral Trading System in 2013 The Current State of Affairs & Expectations for the Short Term Bipul Chatterjee Deputy Executive Director Outline State of Play: 8 th WTO Ministerial Conference Elements
Multilateral Trading System in 2013 The Current State of Affairs & Expectations for the Short Term Bipul Chatterjee Deputy Executive Director Outline State of Play: 8 th WTO Ministerial Conference Elements
FAIR OR FRAUD: HAS THE PROTOCOL AMENDING TRIPS FLOURISHED OR FAILED?
 FAIR OR FRAUD: HAS THE PROTOCOL AMENDING TRIPS FLOURISHED OR FAILED? SIOBHÁN ELIZABETH STADE MURILLO 1 I. INTRODUCTION Global health has been a central concern in both the domestic and international community
FAIR OR FRAUD: HAS THE PROTOCOL AMENDING TRIPS FLOURISHED OR FAILED? SIOBHÁN ELIZABETH STADE MURILLO 1 I. INTRODUCTION Global health has been a central concern in both the domestic and international community
Health 2020: Foreign policy and health
 Sector brief on Foreign affairs July 2015 Health 2020: Foreign policy and health Synergy between sectors: ensuring global health policy coherence Summary The Health 2020 policy framework has been adopted
Sector brief on Foreign affairs July 2015 Health 2020: Foreign policy and health Synergy between sectors: ensuring global health policy coherence Summary The Health 2020 policy framework has been adopted
Introduction Tackling EU Free Trade Agreements
 1 This paper forms part of a series of eight briefings on the European Union s approach to Free Trade. It aims to explain EU policies, procedures and practices to those interested in supporting developing
1 This paper forms part of a series of eight briefings on the European Union s approach to Free Trade. It aims to explain EU policies, procedures and practices to those interested in supporting developing
Answers to the QUESTIONNAIRE on Global Health
 Answers to the QUESTIONNAIRE on Global Health Africa Europe Faith and Justice Network wants to THANK the European Commission for the effort to propose a Communication on Global Health where the input of
Answers to the QUESTIONNAIRE on Global Health Africa Europe Faith and Justice Network wants to THANK the European Commission for the effort to propose a Communication on Global Health where the input of
Second medical use or indication claims. Mr. Antonio Ray ORTIGUERA Angara Abello Concepcion Regala & Cruz Law Offices Philippines
 Question Q238 National Group: Title: Contributors: Reporter within Working Committee: PHILIPPINES Second medical use or indication claims Mr. Alex Ferdinand FIDER Mr. Antonio Ray ORTIGUERA Angara Abello
Question Q238 National Group: Title: Contributors: Reporter within Working Committee: PHILIPPINES Second medical use or indication claims Mr. Alex Ferdinand FIDER Mr. Antonio Ray ORTIGUERA Angara Abello
Development Goals and Strategies
 BEG_i-144.qxd 6/10/04 1:47 PM Page 123 17 Development Goals and Strategies Over the past several decades some developing countries have achieved high economic growth rates, significantly narrowing the
BEG_i-144.qxd 6/10/04 1:47 PM Page 123 17 Development Goals and Strategies Over the past several decades some developing countries have achieved high economic growth rates, significantly narrowing the
Geographical Indications: Implications for Africa. By Catherine Grant For the Trade Law Centre of Southern Africa
 Geographical Indications: Implications for Africa By Catherine Grant For the Trade Law Centre of Southern Africa Introduction The issue of geographical indications (GIs) has been around for many years
Geographical Indications: Implications for Africa By Catherine Grant For the Trade Law Centre of Southern Africa Introduction The issue of geographical indications (GIs) has been around for many years
APEC Study Center Consortium 2014 Qingdao, China. Topic I New Trend of Asia-Pacific Economic Integration INTER-BLOC COMMUNICATION
 APEC Study Center Consortium 2014 Qingdao, China Tatiana Flegontova Maria Ptashkina Topic I New Trend of Asia-Pacific Economic Integration INTER-BLOC COMMUNICATION Abstract: Asia-Pacific is one of the
APEC Study Center Consortium 2014 Qingdao, China Tatiana Flegontova Maria Ptashkina Topic I New Trend of Asia-Pacific Economic Integration INTER-BLOC COMMUNICATION Abstract: Asia-Pacific is one of the
